Hyaluronic Acid/Bone Substitute Complex Implanted on Chick Embryo Chorioallantoic Membrane Induces Osteoblastic Differentiation and Angiogenesis, but not Inflammation
- PMID: 30572565
- PMCID: PMC6320888
- DOI: 10.3390/ijms19124119
Hyaluronic Acid/Bone Substitute Complex Implanted on Chick Embryo Chorioallantoic Membrane Induces Osteoblastic Differentiation and Angiogenesis, but not Inflammation
Abstract
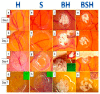
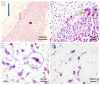
Microscopic and molecular events related to alveolar ridge augmentation are less known because of the lack of experimental models and limited molecular markers used to evaluate this process. We propose here the chick embryo chorioallantoic membrane (CAM) as an in vivo model to study the interaction between CAM and bone substitutes (B) combined with hyaluronic acid (BH), saline solution (BHS and BS, respectively), or both, aiming to point out the microscopic and molecular events assessed by Runt-related transcription factor 2 (RUNX 2), osteonectin (SPARC), and Bone Morphogenic Protein 4 (BMP4). The BH complex induced osteoprogenitor and osteoblastic differentiation of CAM mesenchymal cells, certified by the RUNX2 +, BMP4 +, and SPARC + phenotypes capable of bone matrix synthesis and mineralization. A strong angiogenic response without inflammation was detected on microscopic specimens of the BH combination compared with an inflammatory induced angiogenesis for the BS and BHS combinations. A multilayered organization of the BH complex grafted on CAM was detected with a differential expression of RUNX2, BMP4, and SPARC. The BH complex induced CAM mesenchymal cells differentiation through osteoblastic lineage with a sustained angiogenic response not related with inflammation. Thus, bone granules resuspended in hyaluronic acid seem to be the best combination for a proper non-inflammatory response in alveolar ridge augmentation. The CAM model allows us to assess the early events of the bone substitutes⁻mesenchymal cells interaction related to osteoblastic differentiation, an important step in alveolar ridge augmentation.
Keywords: BMP4; RUNX 2; SPARC; bone substitutes; chorioallantoic membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Establishment of an in ovo chick embryo yolk sac membrane (YSM) assay for pilot screening of potential angiogenic and anti-angiogenic agents.Cell Biol Int. 2018 Nov;42(11):1474-1483. doi: 10.1002/cbin.11051. Epub 2018 Sep 11. Cell Biol Int. 2018. PMID: 30136736
-
[Choline promotes angiogenesis in chick embryo chorioallantoic membrane].Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2013 May;29(3):229-31. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2013. PMID: 23940955 Chinese.
-
CD200 expression in human cultured bone marrow mesenchymal stem cells is induced by pro-osteogenic and pro-inflammatory cues.J Cell Mol Med. 2016 Apr;20(4):655-65. doi: 10.1111/jcmm.12752. Epub 2016 Jan 16. J Cell Mol Med. 2016. PMID: 26773707 Free PMC article.
-
The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems.Adv Drug Deliv Rev. 2007 Sep 30;59(11):1162-76. doi: 10.1016/j.addr.2007.04.019. Epub 2007 Aug 16. Adv Drug Deliv Rev. 2007. PMID: 17870202 Review.
-
Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis.Int Rev Cell Mol Biol. 2008;270:181-224. doi: 10.1016/S1937-6448(08)01405-6. Int Rev Cell Mol Biol. 2008. PMID: 19081537 Review.
Cited by
-
Biological molecules in dental applications: hyaluronic acid as a companion biomaterial for diverse dental applications.Heliyon. 2020 Apr 6;6(4):e03722. doi: 10.1016/j.heliyon.2020.e03722. eCollection 2020 Apr. Heliyon. 2020. PMID: 32280803 Free PMC article. Review.
-
Polymer-Based Carriers in Dental Local Healing-Review and Future Challenges.Materials (Basel). 2021 Jul 14;14(14):3948. doi: 10.3390/ma14143948. Materials (Basel). 2021. PMID: 34300865 Free PMC article. Review.
-
Angiogenesis-Inflammation Cross Talk in Diabetic Retinopathy: Novel Insights From the Chick Embryo Chorioallantoic Membrane/Human Vitreous Platform.Front Immunol. 2020 Sep 29;11:581288. doi: 10.3389/fimmu.2020.581288. eCollection 2020. Front Immunol. 2020. PMID: 33117388 Free PMC article. Review.
-
The Chick Embryo Chorioallantoic Membrane (CAM) Assay: A Novel Experimental Model in Dental Research.Cureus. 2024 Nov 29;16(11):e74714. doi: 10.7759/cureus.74714. eCollection 2024 Nov. Cureus. 2024. PMID: 39655138 Free PMC article. Review.
-
Does Platelet-Rich Fibrin Enhance the Early Angiogenetic Potential of Different Bone Substitute Materials? An In Vitro and In Vivo Analysis.Biomedicines. 2021 Jan 10;9(1):61. doi: 10.3390/biomedicines9010061. Biomedicines. 2021. PMID: 33435244 Free PMC article.
References
-
- Tirone F., Salzano S. Esthetic treatment of alveolar ridge atrophy in the anterior maxilla via connective tissue graft performed simultaneously with implant placement: A three-case series. Quintessence Int. 2018;49:801–807. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

