Contemporary Zika Virus Isolates Induce More dsRNA and Produce More Negative-Strand Intermediate in Human Astrocytoma Cells
- PMID: 30572570
- PMCID: PMC6316034
- DOI: 10.3390/v10120728
Contemporary Zika Virus Isolates Induce More dsRNA and Produce More Negative-Strand Intermediate in Human Astrocytoma Cells
Abstract
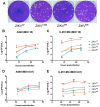
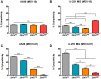
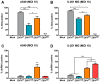
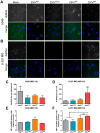
The recent emergence and rapid geographic expansion of Zika virus (ZIKV) poses a significant challenge for public health. Although historically causing only mild febrile illness, recent ZIKV outbreaks have been associated with more severe neurological complications, such as Guillain-Barré syndrome and fetal microcephaly. Here we demonstrate that two contemporary (2015) ZIKV isolates from Puerto Rico and Brazil may have increased replicative fitness in human astrocytoma cells. Over a single infectious cycle, the Brazilian isolate replicates to higher titers and induces more severe cytopathic effects in human astrocytoma cells than the historical African reference strain or an early Asian lineage isolate. In addition, both contemporary isolates induce significantly more double-stranded RNA in infected astrocytoma cells, despite similar numbers of infected cells across isolates. Moreover, when we quantified positive- and negative-strand viral RNA, we found that the Asian lineage isolates displayed substantially more negative-strand replicative intermediates than the African lineage isolate in human astrocytoma cells. However, over multiple rounds of infection, the contemporary ZIKV isolates appear to be impaired in cell spread, infecting a lower proportion of cells at a low MOI despite replicating to similar or higher titers. Taken together, our data suggests that contemporary ZIKV isolates may have evolved mechanisms that allow them to replicate with increased efficiency in certain cell types, thereby highlighting the importance of cell-intrinsic factors in studies of viral replicative fitness.
Keywords: Zika virus; astrocytomas; dsRNA; flavivirus; viral fitness.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Envelope Protein Glycosylation Mediates Zika Virus Pathogenesis.J Virol. 2019 May 29;93(12):e00113-19. doi: 10.1128/JVI.00113-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30944176 Free PMC article.
-
African-Lineage Zika Virus Replication Dynamics and Maternal-Fetal Interface Infection in Pregnant Rhesus Macaques.J Virol. 2021 Jul 26;95(16):e0222020. doi: 10.1128/JVI.02220-20. Epub 2021 Jul 26. J Virol. 2021. PMID: 34076485 Free PMC article.
-
Comparative Analysis of African and Asian Lineage-Derived Zika Virus Strains Reveals Differences in Activation of and Sensitivity to Antiviral Innate Immunity.J Virol. 2019 Jun 14;93(13):e00640-19. doi: 10.1128/JVI.00640-19. Print 2019 Jul 1. J Virol. 2019. PMID: 31019057 Free PMC article.
-
Zika Virus: Emergence, Phylogenetics, Challenges, and Opportunities.ACS Infect Dis. 2016 Nov 11;2(11):763-772. doi: 10.1021/acsinfecdis.6b00161. Epub 2016 Oct 12. ACS Infect Dis. 2016. PMID: 27704772 Review.
-
Functional RNA during Zika virus infection.Virus Res. 2018 Aug 2;254:41-53. doi: 10.1016/j.virusres.2017.08.015. Epub 2017 Aug 31. Virus Res. 2018. PMID: 28864425 Review.
Cited by
-
An overview of the current medical literature on Zika virus.Biophys Rev. 2020 Oct;12(5):1133-1138. doi: 10.1007/s12551-020-00748-8. Epub 2020 Sep 2. Biophys Rev. 2020. PMID: 32880054 Free PMC article.
-
Explaining Pathogenicity of Congenital Zika and Guillain-Barré Syndromes: Does Dysregulation of RNA Editing Play a Role?Bioessays. 2019 Jun;41(6):e1800239. doi: 10.1002/bies.201800239. Epub 2019 May 20. Bioessays. 2019. PMID: 31106880 Free PMC article.
-
A zebrafish-based in vivo model of Zika virus infection unveils alterations of the glutamatergic neuronal development and NS4A as a key viral determinant of neuropathogenesis.PLoS Pathog. 2024 Dec 2;20(12):e1012756. doi: 10.1371/journal.ppat.1012756. eCollection 2024 Dec. PLoS Pathog. 2024. PMID: 39621753 Free PMC article.
-
Profound downregulation of neural transcription factor Npas4 and Nr4a family in fetal mice neurons infected with Zika virus.PLoS Negl Trop Dis. 2021 May 28;15(5):e0009425. doi: 10.1371/journal.pntd.0009425. eCollection 2021 May. PLoS Negl Trop Dis. 2021. PMID: 34048439 Free PMC article.
-
The ZIKA Virus Delays Cell Death Through the Anti-Apoptotic Bcl-2 Family Proteins.Cells. 2019 Oct 29;8(11):1338. doi: 10.3390/cells8111338. Cells. 2019. PMID: 31671831 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

