Development of a recombinase polymerase amplification combined with a lateral flow dipstick assay for rapid detection of the Mycoplasma bovis
- PMID: 30572884
- PMCID: PMC6302395
- DOI: 10.1186/s12917-018-1703-x
Development of a recombinase polymerase amplification combined with a lateral flow dipstick assay for rapid detection of the Mycoplasma bovis
Abstract
Background: Mycoplasma bovis (M. bovis) is a major etiological agent of bovine mycoplasmosis around the world. Point-of-care testing in the field is lacking owing to the requirement for a simple, robust field applicable test that does not require professional laboratory equipment. The recombinase polymerase amplification (RPA) technique has become a promising isothermal DNA amplify assay for use in rapid and low-resource diagnostics.
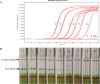
Results: Here, a method for specific detection of M. bovis DNA was established, which was RPA combined with lateral flow dipstick (LFD). First, the analytical specificity and sensitivity of the RPA primer and LF-probe sets were evaluated. The assay successfully detected M. bovis DNA in 30 min at 39 °C, with detection limit of 20 copies per reaction, which it was compared the real-time quantitative PCR (qPCR) assay. This method was specific because it did not detect a selection of other bacterial pathogens in cattle. Both qPCR and RPA-LFD assays were used to detect M. bovis 442 field samples from 42 different dairy farms in Shandong Province of China, also the established RPA-LFD assay obtained 99.00% sensitivity, 95.61% specificity, and 0.902 kappa coefficient compared with the qPCR.
Conclusions: To the author's knowledge, this is the first report using an RPA-FLD assay to visualise and detect M. bovis. Comparative analysis with qPCR indicates the potential of this assay for rapid diagnosis of bovine mycoplasmosis in resource limited settings.
Keywords: Isothermal nucleic acid amplification; Lateral flow dipstick; Mycoplasma bovis; Rapid and visual detection; Recombinase polymerase amplification.
Conflict of interest statement
Ethics approval and consent to participate
Experimental protocols for acquiring cattle clinical samples were performed in strict accordance with the Chinese Regulations of Laboratory Animals (Ministry of Science and Technology of People’s Republic of China, 20,110,108), and the animal study proposal was approved by Shandong Normal University Animal Care and Use Committee (approval No. 20160901).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

