Saccharomyces cerevisiae morphological changes and cytokinesis arrest elicited by hypoxia during scale-up for production of therapeutic recombinant proteins
- PMID: 30572885
- PMCID: PMC6300885
- DOI: 10.1186/s12934-018-1044-2
Saccharomyces cerevisiae morphological changes and cytokinesis arrest elicited by hypoxia during scale-up for production of therapeutic recombinant proteins
Abstract
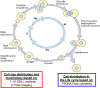
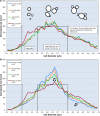
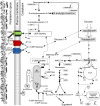
Background: Scaling up of bioprocesses represents a crucial step in the industrial production of biologicals. However, our knowledge about the impact of scale-up on the organism's physiology and function is still incomplete. Our previous studies have suggested the existence of morphological changes during the scale-up of a yeast (Saccharomyces cerevisiae) fermentation process as inferred from the volume fraction occupied by yeast cells and exometabolomics analyses. In the current study, we noticed cell morphology changes during scale-up of a yeast fermentation process from bench (10 L) to industrial scale (10,000 L). We hypothesized that hypoxia observed during scale-up partially impaired the availability of N-acetyl-glucosamine, a precursor of chitin synthesis, a key polysaccharide component of yeast mother-daughter neck formation.
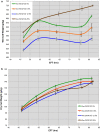
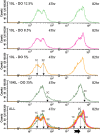
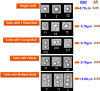
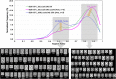
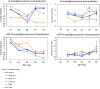
Results: Using a combination of flow cytometry with two high throughput cell imaging technologies, Vi-CELL and Flow Imaging, we found changes in the distribution of cell size and morphology as a function of process duration at the industrial scale of the production process. At the end of run, concomitantly with lowest levels of dissolved oxygen (DO), we detected an increase in cell subpopulations exhibiting low aspect ratio corresponding to morphologies exhibited by large-single-budded and multi-budded cells, reflecting incomplete cytokinesis at the M phase of the yeast mitotic cycle. Metabolomics from the intracellular milieu pointed to an impaired supply of precursors for chitin biosynthesis likely affecting the septum formation between mother and daughter and cytokinesis. Inducing hypoxia at the 10 L bench scale by varying DO levels, confirmed the existence and impact of hypoxic conditions on yeast cell size and morphology observed at the industrial scale.
Conclusions: We conclude that the observed increments in wet cell weight at the industrial scale correspond to morphological changes characterized by the large diameter and low aspect ratio exhibited by cell subpopulations comprising large single-budded and multi-budded cells. These changes are consistent with impairment of cytokinesis triggered by hypoxia as indicated by experiments mimicking this condition at DO 5% and 10 L scale. Mechanistically, hypoxia impairs N-acetyl-glucosamine availability, a key precursor of chitin synthesis.
Keywords: Cell wall; Cytokinesis; Fermentation bioprocess; Hypoxia; Metabolomics; Mitosis; Saccharomyces cerevisiae; Scale-up.
Figures
References
-
- Aon JC, Sun J, Leighton J, Appelbaum E. Hypoxia-elicited impairment of cell wall integrity, glycosylation precursors synthesis, and growth in scaled-up high-cell density fed-batch cultures of Saccharomyces cerevisiae. Microb Cell Fact. 2016;15:142. doi: 10.1186/s12934-016-0542-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

