Developing Gram-negative bacteria for the secretion of heterologous proteins
- PMID: 30572895
- PMCID: PMC6302416
- DOI: 10.1186/s12934-018-1041-5
Developing Gram-negative bacteria for the secretion of heterologous proteins
Abstract
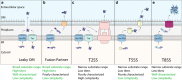
Gram-negative bacteria are attractive hosts for recombinant protein production because they are fast growing, easy to manipulate, and genetically stable in large cultures. However, the utility of these microbes would expand if they also could secrete the product at commercial scales. Secretion of biotechnologically relevant proteins into the extracellular medium increases product purity from cell culture, decreases downstream processing requirements, and reduces overall cost. Thus, researchers are devoting significant attention to engineering Gram-negative bacteria to secrete recombinant proteins to the extracellular medium. Secretion from these bacteria operates through highly specialized systems, which are able to translocate proteins from the cytosol to the extracellular medium in either one or two steps. Building on past successes, researchers continue to increase the secretion efficiency and titer through these systems in an effort to make them viable for industrial production. Efforts include modifying the secretion tags required for recombinant protein secretion, developing methods to screen or select rapidly for clones with higher titer or efficiency, and improving reliability and robustness of high titer secretion through genetic manipulations. An additional focus is the expression of secretion machineries from pathogenic bacteria in the "workhorse" of biotechnology, Escherichia coli, to reduce handling of pathogenic strains. This review will cover recent advances toward the development of high-expressing, high-secreting Gram-negative production strains.
Keywords: Bacterial secretion systems; Protein secretion; Recombinant protein.
Figures

References
-
- Sarrouh B, Santos TM, Miyoshi A, Dias R, Azevedo V. Up-to-date insight on industrial enzymes applications and global market. J Bioprocess Biotechn. 2012;4:002.
-
- Zorko M, Jerala R. Production of recombinant antimicrobial peptides in bacteria. In: Antimicrobial peptides: methods and protocols. Giuliani A, Rinaldi AC, eds. 2010. p. 61–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

