Study of Anti-Malarials in Incomplete Lupus Erythematosus (SMILE): study protocol for a randomized controlled trial
- PMID: 30572906
- PMCID: PMC6302430
- DOI: 10.1186/s13063-018-3076-7
Study of Anti-Malarials in Incomplete Lupus Erythematosus (SMILE): study protocol for a randomized controlled trial
Abstract
Background: Onset of systemic lupus erythematosus (SLE) is preceded by a preclinical phase characterized by expression of autoantibodies and nonspecific clinical symptoms. Hydroxychloroquine is a treatment for lupus that is widely used based on longstanding experience and a very good safety profile. Existing data suggest that treatment with hydroxychloroquine may postpone the onset of disease. However, prospective studies that prove and quantify the efficacy of hydroxychloroquine in the preclinical phase of lupus have not been done. This study will test the hypothesis that early hydroxychloroquine use can prevent accumulation of clinical abnormalities and modify immune responses that define SLE.
Methods: A randomized, double-blind, placebo-controlled trial of hydroxychloroquine vs placebo will be conducted. Participants will have incomplete lupus erythematosus as defined by the presence of antinuclear antibody (ANA) positivity at a titer of 1:80 or greater, as well as one or two additional criteria from the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria. The age range will be 15-45 years and the treatment phase will be 96 weeks. The primary endpoint will be the increase in the number of features of SLE defined by the 2012 SLICC classification schema. Secondary outcomes will include the proportion of participants who transition to a classification of SLE as defined by SLICC criteria.
Discussion: A major challenge for improving therapies in patients with SLE is early detection of disease. The ANA test that is widely used to screen for SLE has low specificity and interpretation of its significance is challenging. The Study of Anti-Malarials in Incomplete Lupus Erythematosus (SMILE) trial will provide insights into the appropriate target population for intervention, and will assess whether hydroxychloroquine can slow progression as measured by the accumulation of criteria. Ophthalmologic safety in this population will be assessed. The study will investigate candidate biomarkers that will guide treatment decisions and will accumulate a specimen biobank that will be available to the lupus research community for further in-depth mechanistic studies. This trial is a first step toward testing the feasibility of disease prevention strategies in SLE.
Trial registration: ClinicalTrials.gov, NCT 03030118 . Registered on 24 January 2017.
Trial registration: ClinicalTrials.gov NCT03030118.
Keywords: Hydroxychloroquine; Incomplete lupus; Prevention; Randomized clinical trial; Systemic lupus erythematosus; Undifferentiated connective tissue disease.
Conflict of interest statement
Ethics approval and consent to participate
This study protocol has been approved by the Institutional Review Boards of each institution: Penn State MS Hershey Medical Center, University of Texas Southwestern Medical Center, Oklahoma Medical Research Foundation, Cedars-Sinai Medical Center and Medical University of South Carolina.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

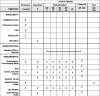
References
-
- Munroe ME, Young KA, Kamen DL, Guthridge JM, Niewold TB, Costenbader KH, et al. Discerning risk of disease transition in relatives of systemic lupus erythematosus patients utilizing soluble mediators and clinical features. Arthritis Rheumatol. 2017;69(3):630–642. doi: 10.1002/art.40004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U54 GM104938/GM/NIGMS NIH HHS/United States
- UL1 TR001105/TR/NCATS NIH HHS/United States
- U01 AR071077/AR/NIAMS NIH HHS/United States
- P30 AR053483/AR/NIAMS NIH HHS/United States
- U34 AR067392/AR/NIAMS NIH HHS/United States
- P30 AR073750/AR/NIAMS NIH HHS/United States
- U34AR067392/National Institute of Arthritis and Musculoskeletal and Skin Diseases
- UL1TR001105/National Center for Advancing Translational Sciences
- U54104938/National Institute of General Medical Sciences
- R01072401/National Institute of Arthritis and Musculoskeletal and Skin Diseases
- U01AR071077/National Institute of Arthritis and Musculoskeletal and Skin Diseases
- P30AR053483/National Institute of Arthritis and Musculoskeletal and Skin Diseases
- R01 AR072401/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

