Acid-Induced Downregulation of ASS1 Contributes to the Maintenance of Intracellular pH in Cancer
- PMID: 30573518
- PMCID: PMC6359973
- DOI: 10.1158/0008-5472.CAN-18-1062
Acid-Induced Downregulation of ASS1 Contributes to the Maintenance of Intracellular pH in Cancer
Abstract
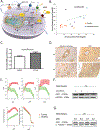
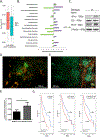
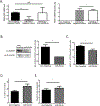
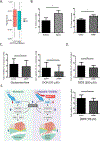
Downregulation of the urea cycle enzyme argininosuccinate synthase (ASS1) by either promoter methylation or by HIF1α is associated with increased metastasis and poor prognosis in multiple cancers. We have previously shown that in normoxic conditions, ASS1 downregulation facilitates cancer cell proliferation by increasing aspartate availability for pyrimidine synthesis by the enzyme complex CAD. Here we report that in hypoxia, ASS1 expression in cancerous cells is downregulated further by HIF1α-mediated induction of miR-224-5p, making the cells more invasive and dependent on upstream substrates of ASS1 for survival. ASS1 was downregulated under acidic conditions, and ASS1-depleted cancer cells maintained a higher intracellular pH (pHi), depended less on extracellular glutamine, and displayed higher glutathione levels. Depletion of substrates of urea cycle enzymes in ASS1-deficient cancers decreased cancer cell survival. Thus, ASS1 levels in cancer are differentially regulated in various environmental conditions to metabolically benefit cancer progression. Understanding these alterations may help uncover specific context-dependent cancer vulnerabilities that may be targeted for therapeutic purposes. SIGNIFICANCE: Cancer cells in an acidic or hypoxic environment downregulate the expression of the urea cycle enzyme ASS1, which provides them with a redox and pH advantage, resulting in better survival.Graphical Abstract: http://cancerres.aacrjournals.org/content/canres/79/3/518/F1.large.jpg.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest
Figures

References
-
- Quinonez SC, Thoene JG. Citrullinemia Type I 2014.
-
- Thoene JG. Citrullinemia Type I In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews Seattle (WA: )2004.
-
- Kobayashi E, Masuda M, Nakayama R, Ichikawa H, Satow R, Shitashige M, et al. Reduced argininosuccinate synthetase is a predictive biomarker for the development of pulmonary metastasis in patients with osteosarcoma. Mol Cancer Ther 2010;9(3):535–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

