Recovery of left ventricular function following in vivo reexpression of cardiac myosin binding protein C
- PMID: 30573635
- PMCID: PMC6314388
- DOI: 10.1085/jgp.201812238
Recovery of left ventricular function following in vivo reexpression of cardiac myosin binding protein C
Abstract
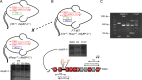
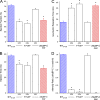
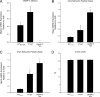
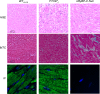
The loss of cardiac myosin binding protein C (cMyBP-C) results in left ventricular dilation, cardiac hypertrophy, and impaired ventricular function in both constitutive and conditional cMyBP-C knockout (MYBPC3 null) mice. It remains unclear whether the structural and functional phenotypes expressed in the MYBPC3 null mouse are reversible, which is an important question, since reduced expression of cMyBP-C is an important cause of hypertrophic cardiomyopathy in humans. To investigate this question, we generated a cardiac-specific transgenic mouse model using a Tet-Off inducible system to permit the controlled expression of WT cMyBP-C on the MYBPC3 null background. Functional Tet-Off mice expressing WT cMyBP-C (FT-WT) were generated by crossing tetracycline transactivator mice with responder mice carrying the WT cMyBP-C transgene. Prior to dietary doxycycline administration, cMyBP-C was expressed at normal levels in FT-WT myocardium, which exhibited similar levels of steady-state force and in vivo left ventricular function as WT mice. Introduction of dietary doxycycline for four weeks resulted in a partial knockdown of cMyBP-C expression and commensurate impairment of systolic and diastolic function to levels approaching those observed in MYBPC 3 null mice. Subsequent withdrawal of doxycycline from the diet resulted in the reexpression of cMyBP-C to levels comparable to those observed in WT mice, along with near-complete recovery of in vivo ventricular function. These results show that the cardiac phenotypes associated with MYBPC3 null mice are reversible. Our work also validates the use of the Tet-Off inducible system as a means to study the mechanisms underlying hypertrophic cardiomyopathy.
© 2018 Giles et al.
Figures
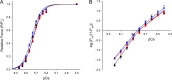
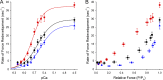
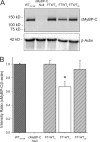
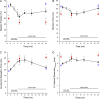
Similar articles
-
Dissociation of structural and functional phenotypes in cardiac myosin-binding protein C conditional knockout mice.Circulation. 2012 Sep 4;126(10):1194-205. doi: 10.1161/CIRCULATIONAHA.111.089219. Epub 2012 Jul 24. Circulation. 2012. PMID: 22829020 Free PMC article.
-
Altered C10 domain in cardiac myosin binding protein-C results in hypertrophic cardiomyopathy.Cardiovasc Res. 2019 Dec 1;115(14):1986-1997. doi: 10.1093/cvr/cvz111. Cardiovasc Res. 2019. PMID: 31050699 Free PMC article.
-
Comparison of the effects of a truncating and a missense MYBPC3 mutation on contractile parameters of engineered heart tissue.J Mol Cell Cardiol. 2016 Aug;97:82-92. doi: 10.1016/j.yjmcc.2016.03.003. Epub 2016 Apr 22. J Mol Cell Cardiol. 2016. PMID: 27108529
-
The mechanics of the heart: zooming in on hypertrophic cardiomyopathy and cMyBP-C.FEBS Lett. 2022 Mar;596(6):703-746. doi: 10.1002/1873-3468.14301. Epub 2022 Feb 28. FEBS Lett. 2022. PMID: 35224729 Review.
-
Cardiac myosin-binding protein C: hypertrophic cardiomyopathy mutations and structure-function relationships.Pflugers Arch. 2014 Feb;466(2):201-6. doi: 10.1007/s00424-013-1400-3. Epub 2013 Nov 17. Pflugers Arch. 2014. PMID: 24240729 Review.
Cited by
-
Modeling the effects of thin filament near-neighbor cooperative interactions in mammalian myocardium.J Gen Physiol. 2025 Mar 3;157(2):e202413582. doi: 10.1085/jgp.202413582. Epub 2025 Jan 27. J Gen Physiol. 2025. PMID: 39869069 Free PMC article.
-
Toward an understanding of the regulation of myofibrillar function.J Gen Physiol. 2019 Jan 7;151(1):1-2. doi: 10.1085/jgp.201812288. Epub 2018 Dec 21. J Gen Physiol. 2019. PMID: 30578329 Free PMC article.
-
The contribution of N-terminal truncated cMyBPC to in vivo cardiac function.J Gen Physiol. 2023 Jun 5;155(6):e202213318. doi: 10.1085/jgp.202213318. Epub 2023 Apr 17. J Gen Physiol. 2023. PMID: 37067542 Free PMC article.
-
Cooperative mechanisms underlie differences in myocardial contractile dynamics between large and small mammals.J Gen Physiol. 2023 Nov 6;155(11):e202213315. doi: 10.1085/jgp.202213315. Epub 2023 Sep 19. J Gen Physiol. 2023. PMID: 37725091 Free PMC article.
-
Spatial and Functional Distribution of MYBPC3 Pathogenic Variants and Clinical Outcomes in Patients With Hypertrophic Cardiomyopathy.Circ Genom Precis Med. 2020 Oct;13(5):396-405. doi: 10.1161/CIRCGEN.120.002929. Epub 2020 Aug 25. Circ Genom Precis Med. 2020. PMID: 32841044 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

