Prefrontal-Bed Nucleus Circuit Modulation of a Passive Coping Response Set
- PMID: 30573644
- PMCID: PMC6381256
- DOI: 10.1523/JNEUROSCI.1421-18.2018
Prefrontal-Bed Nucleus Circuit Modulation of a Passive Coping Response Set
Abstract
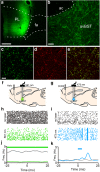
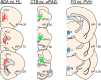
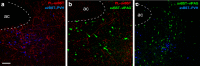
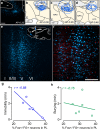
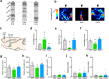
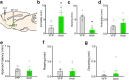
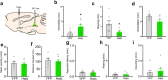
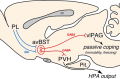
One of the challenges facing neuroscience entails localization of circuits and mechanisms accounting for how multiple features of stress responses are organized to promote survival during adverse experiences. The rodent medial prefrontal cortex (mPFC) is generally regarded as a key site for cognitive and affective information processing, and the anteroventral bed nuclei of the stria terminalis (avBST) integrates homeostatic information from a variety of sources, including the mPFC. Thus, we proposed that the mPFC is capable of generating multiple features (endocrine, behavioral) of adaptive responses via its influence over the avBST. To address this possibility, we first optogenetically inhibited input to avBST from the rostral prelimbic cortical region of mPFC and observed concurrent increases in immobility and hypothalamo-pituitary-adrenal (HPA) output in male rats during tail suspension, whereas photostimulation of this pathway decreased immobility during the same challenge. Anatomical tracing experiments confirmed projections from the rostral prelimbic subfield to separate populations of avBST neurons, and from these to HPA effector neurons in the paraventricular hypothalamic nucleus, and to aspects of the midbrain periaqueductal gray that coordinate passive defensive behaviors. Finally, stimulation and inhibition of the prelimbic-avBST pathway, respectively, decreased and increased passive coping in the shock-probe defensive burying test, without having any direct effect on active coping (burying) behavior. These results define a new neural substrate in the coordination of a response set that involves the gating of passive, rather than active, coping behaviors while restraining neuroendocrine activation to optimize adaptation during threat exposure.SIGNIFICANCE STATEMENT The circuits and mechanisms accounting for how multiple features of responses are organized to promote adaptation have yet to be elucidated. Our report identifies a prefrontal-bed nucleus pathway that organizes a response set capable of gating passive coping behaviors while concurrently restraining neuroendocrine activation during exposure to inescapable stressors. These data provide insight into the central organization of how multiple features of responses are integrated to promote adaptation during adverse experiences, and how disruption in one neural pathway may underlie a broad array of maladaptive responses in stress-related psychiatric disorders.
Keywords: HPA; bed nucleus; passive coping; prelimbic; shock probe defensive burying; ventrolateral periaqueductal gray area.
Copyright © 2019 the authors 0270-6474/19/391405-15$15.00/0.
Figures


Similar articles
-
A Prefrontal→Periaqueductal Gray Pathway Differentially Engages Autonomic, Hormonal, and Behavioral Features of the Stress-Coping Response.J Neurosci. 2024 Nov 13;44(46):e0844242024. doi: 10.1523/JNEUROSCI.0844-24.2024. J Neurosci. 2024. PMID: 39313320 Free PMC article.
-
Anteroventral bed nuclei of the stria terminalis neurocircuitry: Towards an integration of HPA axis modulation with coping behaviors - Curt Richter Award Paper 2017.Psychoneuroendocrinology. 2018 Mar;89:239-249. doi: 10.1016/j.psyneuen.2017.12.005. Epub 2017 Dec 24. Psychoneuroendocrinology. 2018. PMID: 29395488 Free PMC article. Review.
-
A Basal Forebrain Site Coordinates the Modulation of Endocrine and Behavioral Stress Responses via Divergent Neural Pathways.J Neurosci. 2016 Aug 17;36(33):8687-99. doi: 10.1523/JNEUROSCI.1185-16.2016. J Neurosci. 2016. PMID: 27535914 Free PMC article.
-
Bed nuclei of the stria terminalis modulate memory consolidation via glucocorticoid-dependent and -independent circuits.Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):8104-8114. doi: 10.1073/pnas.1915501117. Epub 2020 Mar 19. Proc Natl Acad Sci U S A. 2020. PMID: 32193346 Free PMC article.
-
Central circuits mediating patterned autonomic activity during active vs. passive emotional coping.Brain Res Bull. 2000 Sep 1;53(1):95-104. doi: 10.1016/s0361-9230(00)00313-0. Brain Res Bull. 2000. PMID: 11033213 Review.
Cited by
-
Interactions Between Experience, Genotype and Sex in the Development of Individual Coping Strategies.Front Behav Neurosci. 2021 Dec 20;15:785739. doi: 10.3389/fnbeh.2021.785739. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34987364 Free PMC article.
-
Emotional- and cognitive-like responses induced by social defeat stress in male mice are modulated by the BNST, amygdala, and hippocampus.Front Integr Neurosci. 2023 Jun 12;17:1168640. doi: 10.3389/fnint.2023.1168640. eCollection 2023. Front Integr Neurosci. 2023. PMID: 37377628 Free PMC article.
-
Prefrontal modulation of anxiety through a lens of noradrenergic signaling.Front Syst Neurosci. 2023 Apr 17;17:1173326. doi: 10.3389/fnsys.2023.1173326. eCollection 2023. Front Syst Neurosci. 2023. PMID: 37139472 Free PMC article. Review.
-
A prefrontal-bed nucleus of the stria terminalis circuit limits fear to uncertain threat.Elife. 2020 Dec 15;9:e60812. doi: 10.7554/eLife.60812. Elife. 2020. PMID: 33319747 Free PMC article.
-
Coping with the multifaceted and multifunctional role of cortisol in the brain.Neurosci Appl. 2024 Feb 14;3:104047. doi: 10.1016/j.nsa.2024.104047. eCollection 2024. Neurosci Appl. 2024. PMID: 40656063 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
