Cross-Modal Competition: The Default Computation for Multisensory Processing
- PMID: 30573648
- PMCID: PMC6381255
- DOI: 10.1523/JNEUROSCI.1806-18.2018
Cross-Modal Competition: The Default Computation for Multisensory Processing
Abstract
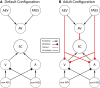
Mature multisensory superior colliculus (SC) neurons integrate information across the senses to enhance their responses to spatiotemporally congruent cross-modal stimuli. The development of this neurotypic feature of SC neurons requires experience with cross-modal cues. In the absence of such experience the response of an SC neuron to congruent cross-modal cues is no more robust than its response to the most effective component cue. This "default" or "naive" state is believed to be one in which cross-modal signals do not interact. The present results challenge this characterization by identifying interactions between visual-auditory signals in male and female cats reared without visual-auditory experience. By manipulating the relative effectiveness of the visual and auditory cross-modal cues that were presented to each of these naive neurons, an active competition between cross-modal signals was revealed. Although contrary to current expectations, this result is explained by a neuro-computational model in which the default interaction is mutual inhibition. These findings suggest that multisensory neurons at all maturational stages are capable of some form of multisensory integration, and use experience with cross-modal stimuli to transition from their initial state of competition to their mature state of cooperation. By doing so, they develop the ability to enhance the physiological salience of cross-modal events thereby increasing their impact on the sensorimotor circuitry of the SC, and the likelihood that biologically significant events will elicit SC-mediated overt behaviors.SIGNIFICANCE STATEMENT The present results demonstrate that the default mode of multisensory processing in the superior colliculus is competition, not non-integration as previously characterized. A neuro-computational model explains how these competitive dynamics can be implemented via mutual inhibition, and how this default mode is superseded by the emergence of cooperative interactions during development.
Keywords: computational modeling; enhancement; inhibition; integration; plasticity; superior colliculus.
Copyright © 2019 the authors 0270-6474/19/391374-12$15.00/0.
Figures

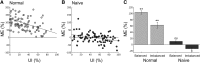





Similar articles
-
Multisensory Plasticity in Superior Colliculus Neurons is Mediated by Association Cortex.Cereb Cortex. 2016 Mar;26(3):1130-7. doi: 10.1093/cercor/bhu295. Epub 2014 Dec 31. Cereb Cortex. 2016. PMID: 25552270 Free PMC article.
-
Incorporating cross-modal statistics in the development and maintenance of multisensory integration.J Neurosci. 2012 Feb 15;32(7):2287-98. doi: 10.1523/JNEUROSCI.4304-11.2012. J Neurosci. 2012. PMID: 22396404 Free PMC article.
-
The normal environment delays the development of multisensory integration.Sci Rep. 2017 Jul 6;7(1):4772. doi: 10.1038/s41598-017-05118-1. Sci Rep. 2017. PMID: 28684852 Free PMC article.
-
Nonvisual influences on visual-information processing in the superior colliculus.Prog Brain Res. 2001;134:143-56. doi: 10.1016/s0079-6123(01)34011-6. Prog Brain Res. 2001. PMID: 11702540 Review.
-
A model of the temporal dynamics of multisensory enhancement.Neurosci Biobehav Rev. 2014 Apr;41:78-84. doi: 10.1016/j.neubiorev.2013.12.003. Epub 2013 Dec 26. Neurosci Biobehav Rev. 2014. PMID: 24374382 Free PMC article. Review.
Cited by
-
Primary sensory map formations reflect unique needs and molecular cues specific to each sensory system.F1000Res. 2019 Mar 27;8:F1000 Faculty Rev-345. doi: 10.12688/f1000research.17717.1. eCollection 2019. F1000Res. 2019. PMID: 30984379 Free PMC article. Review.
-
Noise-rearing precludes the behavioral benefits of multisensory integration.Cereb Cortex. 2023 Feb 7;33(4):948-958. doi: 10.1093/cercor/bhac113. Cereb Cortex. 2023. PMID: 35332919 Free PMC article.
-
Multisensory enhancement of overt behavior requires multisensory experience.Eur J Neurosci. 2021 Jul;54(2):4514-4527. doi: 10.1111/ejn.15315. Epub 2021 Jun 10. Eur J Neurosci. 2021. PMID: 34013578 Free PMC article.
-
Crossmodal interactions in human learning and memory.Front Hum Neurosci. 2023 May 17;17:1181760. doi: 10.3389/fnhum.2023.1181760. eCollection 2023. Front Hum Neurosci. 2023. PMID: 37266327 Free PMC article. Review.
-
Resolution of impaired multisensory processing in autism and the cost of switching sensory modality.Commun Biol. 2022 Jun 30;5(1):601. doi: 10.1038/s42003-022-03519-1. Commun Biol. 2022. PMID: 35773473 Free PMC article.
References
-
- Aslin RN, Alberts JR, Petersen MR (1981) Development of perception: psychobiological perspectives. New York: Academic.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
