Adipose Tissue, Inter-Organ Communication, and the Path to Type 2 Diabetes: The 2016 Banting Medal for Scientific Achievement Lecture
- PMID: 30573674
- PMCID: PMC6302542
- DOI: 10.2337/dbi18-0035
Adipose Tissue, Inter-Organ Communication, and the Path to Type 2 Diabetes: The 2016 Banting Medal for Scientific Achievement Lecture
Abstract
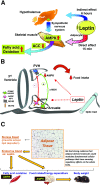
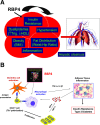
My scientific career has focused on understanding the mechanisms underlying insulin resistance with the goal of developing new strategies to prevent and treat type 2 diabetes. My early studies focused on understanding how insulin promotes glucose transport into adipocytes, a classic model of highly insulin-responsive target cells. When we found changes in adipocyte glucose transport in altered metabolic states, we were highly motivated to understand the consequences of this on whole-body glucose homeostasis. In the late 1980s, when GLUT4, the major insulin-regulated glucose transporter, was identified, my lab observed that it was downregulated in adipocytes but not in skeletal muscle in insulin-resistant states, such as obesity and type 2 diabetes, in humans and rodents. We investigated the role of GLUT4 in adipose tissue and muscle in whole-body insulin sensitivity, making tissue-specific GLUT4-overexpressing and GLUT4 knockout mice. These studies led to the discovery that adipocytes, and specifically glucose transport into adipocytes, regulate whole-body glucose homeostasis. As adipocytes take up relatively little glucose, we investigated the underlying mechanisms. In the 1990s, we performed DNA microarrays on adipose tissue from adipose-specific GLUT4-overexpressing and GLUT4 knockout mice to find reciprocally regulated genes, and we identified several molecules that were not previously known to regulate systemic insulin sensitivity and/or energy balance. More recently, with Alan Saghatelian's lab, we discovered a novel class of lipids with antidiabetes and anti-inflammatory effects. We also investigated the effects of the adipose-secreted hormone, leptin, on insulin sensitivity. We found that the AMP-activated protein kinase (AMPK) pathway mediates leptin's effects on fatty acid oxidation in muscle and also plays a role in leptin's anorexigenic effects in the hypothalamus. These studies transformed AMPK from a "fuel gauge" that regulates energy supply at the cellular level to a sensing and signaling pathway that regulates organismal energy balance. Overall, these studies have expanded our understanding of the multifaceted role of adipose tissue in metabolic health and how adipose dysfunction increases the risk for type 2 diabetes.
© 2018 by the American Diabetes Association.
Figures






Similar articles
-
Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle.Am J Physiol Endocrinol Metab. 2005 Oct;289(4):E551-61. doi: 10.1152/ajpendo.00116.2005. Epub 2005 May 31. Am J Physiol Endocrinol Metab. 2005. PMID: 15928024
-
Lilly lecture 1995. Glucose transport: pivotal step in insulin action.Diabetes. 1996 Nov;45(11):1644-54. doi: 10.2337/diab.45.11.1644. Diabetes. 1996. PMID: 8866574 Review.
-
High level overexpression of glucose transporter-4 driven by an adipose-specific promoter is maintained in transgenic mice on a high fat diet, but does not prevent impaired glucose tolerance.Endocrinology. 1995 Mar;136(3):995-1002. doi: 10.1210/endo.136.3.7867610. Endocrinology. 1995. PMID: 7867610
-
Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver.Nature. 2001 Feb 8;409(6821):729-33. doi: 10.1038/35055575. Nature. 2001. PMID: 11217863
-
Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids.J Intern Med. 2016 Nov;280(5):465-475. doi: 10.1111/joim.12540. Epub 2016 Oct 3. J Intern Med. 2016. PMID: 27699898 Free PMC article. Review.
Cited by
-
Molecular and Cellular Bases of Lipodystrophy Syndromes.Front Endocrinol (Lausanne). 2022 Jan 3;12:803189. doi: 10.3389/fendo.2021.803189. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35046902 Free PMC article. Review.
-
ChREBP-Mediated Regulation of Lipid Metabolism: Involvement of the Gut Microbiota, Liver, and Adipose Tissue.Front Endocrinol (Lausanne). 2020 Dec 3;11:587189. doi: 10.3389/fendo.2020.587189. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33343508 Free PMC article. Review.
-
New Horizons: Next-Generation Insulin Analogues: Structural Principles and Clinical Goals.J Clin Endocrinol Metab. 2022 Mar 24;107(4):909-928. doi: 10.1210/clinem/dgab849. J Clin Endocrinol Metab. 2022. PMID: 34850005 Free PMC article. Review.
-
Hypoxia in Human Obesity: New Insights from Inflammation towards Insulin Resistance-A Narrative Review.Int J Mol Sci. 2024 Sep 11;25(18):9802. doi: 10.3390/ijms25189802. Int J Mol Sci. 2024. PMID: 39337290 Free PMC article. Review.
-
Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation.Foods. 2022 Apr 25;11(9):1232. doi: 10.3390/foods11091232. Foods. 2022. PMID: 35563955 Free PMC article. Review.
References
-
- Ross SR, Graves RA, Spiegelman BM. Targeted expression of a toxin gene to adipose tissue: transgenic mice resistant to obesity. Genes Dev 1993;7:1318–1324 - PubMed
-
- Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem 1993;268:22243–22246 - PubMed
-
- Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab 2005;289:E551–E561 - PubMed
-
- Abel ED, Peroni O, Kim JK, et al. . Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001;409:729–733 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

