Murine hematopoietic stem cell activity is derived from pre-circulation embryos but not yolk sacs
- PMID: 30573729
- PMCID: PMC6302089
- DOI: 10.1038/s41467-018-07769-8
Murine hematopoietic stem cell activity is derived from pre-circulation embryos but not yolk sacs
Abstract
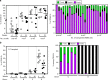
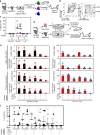
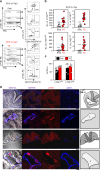
The embryonic site of definitive hematopoietic stem cell (dHSC) origination has been debated for decades. Although an intra-embryonic origin is well supported, the yolk sac (YS) contribution to adult hematopoiesis remains controversial. The same developmental origin makes it difficult to identify specific markers that discern between an intraembryonic versus YS-origin using a lineage trace approach. Additionally, the highly migratory nature of blood cells and the inability of pre-circulatory embryonic cells (i.e., 5-7 somite pairs (sp)) to robustly engraft in transplantation, even after culture, has precluded scientists from properly answering these questions. Here we report robust, multi-lineage and serially transplantable dHSC activity from cultured 2-7sp murine embryonic explants (Em-Ex). dHSC are undetectable in 2-7sp YS explants. Additionally, the engraftment from Em-Ex is confined to an emerging CD31+CD45+c-Kit+CD41- population. In sum, our work supports a model in which the embryo, not the YS, is the major source of lifelong definitive hematopoiesis.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

