Fast animal pose estimation using deep neural networks
- PMID: 30573820
- PMCID: PMC6899221
- DOI: 10.1038/s41592-018-0234-5
Fast animal pose estimation using deep neural networks
Abstract
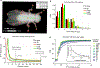
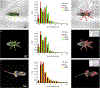
The need for automated and efficient systems for tracking full animal pose has increased with the complexity of behavioral data and analyses. Here we introduce LEAP (LEAP estimates animal pose), a deep-learning-based method for predicting the positions of animal body parts. This framework consists of a graphical interface for labeling of body parts and training the network. LEAP offers fast prediction on new data, and training with as few as 100 frames results in 95% of peak performance. We validated LEAP using videos of freely behaving fruit flies and tracked 32 distinct points to describe the pose of the head, body, wings and legs, with an error rate of <3% of body length. We recapitulated reported findings on insect gait dynamics and demonstrated LEAP's applicability for unsupervised behavioral classification. Finally, we extended the method to more challenging imaging situations and videos of freely moving mice.
Figures




References
-
- Anderson DJ & Perona P Toward a science of computational ethology. Neuron 84, 18–31 (2014). - PubMed
-
- Szigeti B, Stone T & Webb B Inconsistencies in C. elegans behavioural annotation. Preprint at bioRxiv https://www.biorxiv.org/content/early/2016/07/29/066787 (2016).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

