The Emerging Role of Mechanics in Synapse Formation and Plasticity
- PMID: 30574071
- PMCID: PMC6291423
- DOI: 10.3389/fncel.2018.00483
The Emerging Role of Mechanics in Synapse Formation and Plasticity
Abstract
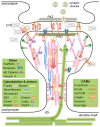
The regulation of synaptic strength forms the basis of learning and memory, and is a key factor in understanding neuropathological processes that lead to cognitive decline and dementia. While the mechanical aspects of neuronal development, particularly during axon growth and guidance, have been extensively studied, relatively little is known about the mechanical aspects of synapse formation and plasticity. It is established that a filamentous actin network with complex spatiotemporal behavior controls the dendritic spine shape and size, which is thought to be crucial for activity-dependent synapse plasticity. Accordingly, a number of actin binding proteins have been identified as regulators of synapse plasticity. On the other hand, a number of cell adhesion molecules (CAMs) are found in synapses, some of which form transsynaptic bonds to align the presynaptic active zone (PAZ) with the postsynaptic density (PSD). Considering that these CAMs are key components of cellular mechanotransduction, two critical questions emerge: (i) are synapses mechanically regulated? and (ii) does disrupting the transsynaptic force balance lead to (or exacerbate) synaptic failure? In this mini review article, I will highlight the mechanical aspects of synaptic structures-focusing mainly on cytoskeletal dynamics and CAMs-and discuss potential mechanoregulation of synapses and its relevance to neurodegenerative diseases.
Keywords: cell adhesion molecules; cytoskeleton; dendritic spine; mechanotransduction; motor proteins; synaptic scaffold proteins.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

