Human Mesenchymal Stem Cell Therapy Reverses Su5416/Hypoxia-Induced Pulmonary Arterial Hypertension in Mice
- PMID: 30574088
- PMCID: PMC6291748
- DOI: 10.3389/fphar.2018.01395
Human Mesenchymal Stem Cell Therapy Reverses Su5416/Hypoxia-Induced Pulmonary Arterial Hypertension in Mice
Abstract
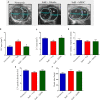
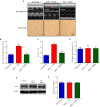
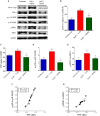
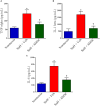
Aims: Pulmonary arterial hypertension (PAH) is a disease characterized by an increase in pulmonary vascular resistance and right ventricular (RV) failure. We aimed to determine the effects of human mesenchymal stem cell (hMSC) therapy in a SU5416/hypoxia (SuH) mice model of PAH. Methods and Results: C57BL/6 mice (20-25 g) were exposure to 4 weeks of hypoxia combined vascular endothelial growth factor receptor antagonism (20 mg/kg SU5416; weekly s.c. injections; PAH mice). Control mice were housed in room air. Following 2 weeks of SuH exposure, we injected 5 × 105 hMSCs cells suspended in 50 μL of vehicle (0.6 U/mL DNaseI in PBS) through intravenous injection in the caudal vein. PAH mice were treated only with vehicle. Ratio between pulmonary artery acceleration time and RV ejection time (PAAT/RVET), measure by echocardiography, was significantly reduced in the PAH mice, compared with controls, and therapy with hMSCs normalized this. Significant muscularization of the PA was observed in the PAH mice and hMSC reduced the number of fully muscularized vessels. RV free wall thickness was higher in PAH animals than in the controls, and a single injection of hMSCs reversed RV hypertrophy. Levels of markers of exacerbated apoptosis, tissue inflammation and damage, cell proliferation and oxidative stress were significantly greater in both lungs and RV tissues from PAH group, compared to controls. hMSC injection in PAH animals normalized the expression of these molecules which are involved with PAH and RV dysfunction development and the state of chronicity. Conclusion: These results indicate that hMSCs therapy represents a novel strategy for the treatment of PAH in the future.
Keywords: apoptosis; cell proliferation; human mesenchymal stem cell; inflammation; pulmonary arterial hypertension.
Figures
Similar articles
-
Therapeutic Benefit of the Association of Lodenafil with Mesenchymal Stem Cells on Hypoxia-induced Pulmonary Hypertension in Rats.Cells. 2020 Sep 18;9(9):2120. doi: 10.3390/cells9092120. Cells. 2020. PMID: 32961896 Free PMC article.
-
Contribution of Impaired Parasympathetic Activity to Right Ventricular Dysfunction and Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension.Circulation. 2018 Feb 27;137(9):910-924. doi: 10.1161/CIRCULATIONAHA.117.027451. Epub 2017 Nov 22. Circulation. 2018. PMID: 29167228
-
Pharmacological Inhibition of mTOR Kinase Reverses Right Ventricle Remodeling and Improves Right Ventricle Structure and Function in Rats.Am J Respir Cell Mol Biol. 2017 Nov;57(5):615-625. doi: 10.1165/rcmb.2016-0364OC. Am J Respir Cell Mol Biol. 2017. PMID: 28679058 Free PMC article.
-
The effects of antiangiogenic compound SU5416 in a rat model of pulmonary arterial hypertension.Respiration. 2011;81(3):253-61. doi: 10.1159/000322011. Epub 2010 Nov 30. Respiration. 2011. PMID: 21116108 Review.
-
Stem and progenitor cell therapy for pulmonary arterial hypertension: effects on the right ventricle (2013 Grover Conference Series).Pulm Circ. 2015 Mar;5(1):73-80. doi: 10.1086/679701. Pulm Circ. 2015. PMID: 25992272 Free PMC article. Review.
Cited by
-
Effects of Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Lung Diseases: Current Status and Future Perspectives.Stem Cell Rev Rep. 2021 Apr;17(2):440-458. doi: 10.1007/s12015-020-10085-8. Epub 2020 Nov 19. Stem Cell Rev Rep. 2021. PMID: 33211245 Free PMC article. Review.
-
Mesenchymal Stem Cell-derived Nanovesicles as a Credible Agent for Therapy of Pulmonary Hypertension.Am J Respir Cell Mol Biol. 2022 Jul;67(1):61-75. doi: 10.1165/rcmb.2021-0415OC. Am J Respir Cell Mol Biol. 2022. PMID: 35507777 Free PMC article.
-
Effect of dose, dosing intervals, and hypoxic stress on the reversal of pulmonary hypertension by mesenchymal stem cell extracellular vesicles.Pulm Circ. 2021 Oct 21;11(4):20458940211046137. doi: 10.1177/20458940211046137. eCollection 2021 Oct-Dec. Pulm Circ. 2021. PMID: 34987768 Free PMC article.
-
Stem cell therapy in pulmonary hypertension: current practice and future opportunities.Eur Respir Rev. 2023 Sep 27;32(169):230112. doi: 10.1183/16000617.0112-2023. Print 2023 Sep 30. Eur Respir Rev. 2023. PMID: 37758272 Free PMC article. Review.
-
The Brazilian compound library (BraCoLi) database: a repository of chemical and biological information for drug design.Mol Divers. 2022 Dec;26(6):3387-3397. doi: 10.1007/s11030-022-10386-9. Epub 2022 Jan 28. Mol Divers. 2022. PMID: 35089481
References
-
- Alencar A. K., Pereira S. L., Da Silva F. E., Mendes L. V., Cunha Vdo M., Lima L. M., et al. (2014). N-acylhydrazone derivative ameliorates monocrotaline-induced pulmonary hypertension through the modulation of adenosine AA2R activity. Int. J. Cardiol. 173 154–162. 10.1016/j.ijcard.2014.02.022 - DOI - PubMed
-
- Badr Eslam R., Croce K., Mangione F. M., Musmann R., Leopold J. A., Mitchell R. N., et al. (2017). Persistence and proliferation of human mesenchymal stromal cells in the right ventricular myocardium after intracoronary injection in a large animal model of pulmonary hypertension. Cytotherapy 19 668–679. 10.1016/j.jcyt.2017.03.002 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

