A Nanostructured Lipid System to Improve the Oral Bioavailability of Ruthenium(II) Complexes for the Treatment of Infections Caused by Mycobacterium tuberculosis
- PMID: 30574128
- PMCID: PMC6291527
- DOI: 10.3389/fmicb.2018.02930
A Nanostructured Lipid System to Improve the Oral Bioavailability of Ruthenium(II) Complexes for the Treatment of Infections Caused by Mycobacterium tuberculosis
Erratum in
-
Corrigendum: A Nanostructured Lipid System to Improve the Oral Bioavailability of Ruthenium(II) Complexes for the Treatment of Infections Caused by Mycobacterium tuberculosis.Front Microbiol. 2019 May 31;10:1213. doi: 10.3389/fmicb.2019.01213. eCollection 2019. Front Microbiol. 2019. PMID: 31214147 Free PMC article.
-
Corrigendum: A Nanostructured Lipid System to Improve the Oral Bioavailability of Ruthenium(II) Complexes for the Treatment of Infections Caused by Mycobacterium tuberculosis.Front Microbiol. 2019 Nov 5;10:2517. doi: 10.3389/fmicb.2019.02517. eCollection 2019. Front Microbiol. 2019. PMID: 31736932 Free PMC article.
Abstract
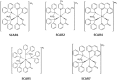
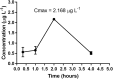
Tuberculosis (TB) is an infectious, airborne disease caused by the bacterium Mycobacterium tuberculosis that mainly affects the lungs. Fortunately, tuberculosis is a curable disease, and in recent years, death rates for this disease have decreased. However, the existence of antibiotic-resistant strains and the occurrence of co-infections with human immunodeficiency virus (HIV), have led to increased mortality in recent years. Another area of concern is that one-third of the world's population is currently infected with M. tuberculosis in its latent state, serving as a potential reservoir for active TB. In an effort to address the failure of current TB drugs, greater attention is being given to the importance of bioinorganic chemistry as an ally in new research into the development of anti-TB drugs. Ruthenium (Ru) is a chemical element that can mimic iron (Fe) in the body. In previous studies involving the following heteroleptic Ru complexes, [Ru(pic)(dppb)(bipy)]PF6 (SCAR1), [Ru(pic)(dppb)(Me-bipy)]PF6 (SCAR2), [Ru(pic)(dppb)(phen)]PF6 (SCAR4), cis-[Ru(pic)(dppe)2]PF6 (SCAR5), and [Ru(pic)(dppe)(phen)]PF6 (SCAR7), we observed excellent anti-TB activity, moderate cell-toxicity, and a lack of oral bioavailability in an in vivo model of these complexes. Therefore, the objective of this study was to evaluate the toxicity and oral bioavailability of these complexes by loading them into a nanostructured lipid system. The nanostructured lipid system was generated using different ratios of surfactant (soybean phosphatidylcholine, Eumulgin®, and sodium oleate), aqueous phase (phosphate buffer with a concentration of 1X and pH 7.4), and oil (cholesterol) to generate a system for the incorporation of Ru(II) compounds. The anti-TB activity of the compounds was determined using a microdilution assay with Resazurin (REMA) against strains of M. tuberculosis H37Rv and clinical isolates resistant. Cytotoxicity assay using J774.A1 cells (ATCC TIB-67) and intra-macrophage activity were performed. The oral bioavailability assay was used to analyze blood collected from female BALB/C mice. Plasma collected from the same mice was analyzed via inductively coupled plasma mass spectrometry (ICP-MS) to quantify the number of Ru ions. The complexes loaded into the nanostructured lipid system maintained in vitro activity and toxicity was found to be reduced compared with the compounds that were not loaded. The complexes showed intra-macrophagic activity and were orally bioavailable.
Keywords: ICP-MS; nanotechnology; oral bioavailability; ruthenium(II) complexes; tuberculosis.
Figures

Similar articles
-
Ruthenium(II) phosphine/diimine/picolinate complexes: inorganic compounds as agents against tuberculosis.Eur J Med Chem. 2011 Oct;46(10):5099-107. doi: 10.1016/j.ejmech.2011.08.023. Epub 2011 Aug 23. Eur J Med Chem. 2011. PMID: 21875763
-
In Vitro Activity of Copper(II) Complexes, Loaded or Unloaded into a Nanostructured Lipid System, against Mycobacterium tuberculosis.Int J Mol Sci. 2016 May 17;17(5):745. doi: 10.3390/ijms17050745. Int J Mol Sci. 2016. PMID: 27196901 Free PMC article.
-
Antiparasitic activities of novel ruthenium/lapachol complexes.J Inorg Biochem. 2014 Jul;136:33-9. doi: 10.1016/j.jinorgbio.2014.03.009. Epub 2014 Mar 27. J Inorg Biochem. 2014. PMID: 24727183
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Tuberculosis in an Aging World.Pathogens. 2022 Sep 26;11(10):1101. doi: 10.3390/pathogens11101101. Pathogens. 2022. PMID: 36297158 Free PMC article. Review.
Cited by
-
In Vitro, In Vivo and In Silico Effectiveness of LASSBio-1386, an N-Acyl Hydrazone Derivative Phosphodiesterase-4 Inhibitor, Against Leishmania amazonensis.Front Pharmacol. 2020 Dec 16;11:590544. doi: 10.3389/fphar.2020.590544. eCollection 2020. Front Pharmacol. 2020. PMID: 33390966 Free PMC article.
-
Breaking barriers: The potential of nanosystems in antituberculosis therapy.Bioact Mater. 2024 May 17;39:106-134. doi: 10.1016/j.bioactmat.2024.05.013. eCollection 2024 Sep. Bioact Mater. 2024. PMID: 38783925 Free PMC article. Review.
References
-
- Allardyce B. C. S., Dyson P. J. (2001). Ruthenium in medicine: current clinical uses and future prospects. Platin. Met. Rev. 45 62–69.
-
- Barbosa M. I. F., Corrêa R. S., Pozzi L. V., de O. Lopes E., Pavan F. R., Leite C. Q. F., et al. (2015). Ruthenium(II) complexes with hydroxypyridinecarboxylates: screening potential metallodrugs against Mycobacterium tuberculosis. Polyhedron 85 376–382.
-
- Batista B. L., Rodrigues J. L., Nunes J. A., de Oliveira Souza V. C., Barbosa F. (2009). Exploiting dynamic reaction cell inductively coupled plasma mass spectrometry (DRC-ICP-MS) for sequential determination of trace elements in blood using a dilute-and-shoot procedure. Anal. Chim. Acta 639 13–18. 10.1016/j.aca.2009.03.016 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

