The Aryl Hydrocarbon Receptor as an Immune-Modulator of Atmospheric Particulate Matter-Mediated Autoimmunity
- PMID: 30574142
- PMCID: PMC6291477
- DOI: 10.3389/fimmu.2018.02833
The Aryl Hydrocarbon Receptor as an Immune-Modulator of Atmospheric Particulate Matter-Mediated Autoimmunity
Abstract
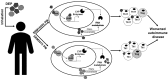
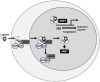
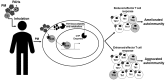
This review examines the current literature on the effects of atmospheric particulate matter (PM) on autoimmune disease and proposes a new role for the aryl hydrocarbon receptor (AHR) as a modulator of T cells in PM-mediated autoimmune disease. There is a significant body of literature regarding the strong epidemiologic correlations between PM exposures and worsened autoimmune diseases. Genetic predispositions account for 30% of all autoimmune disease leaving environmental factors as major contributors. Increases in incidence and prevalence of autoimmune disease have occurred concurrently with an increase in air pollution. Currently, atmospheric PM is considered to be the greatest environmental health risk worldwide. Atmospheric PM is a complex heterogeneous mixture composed of diverse adsorbed organic compounds such as polycyclic aromatic hydrocarbons (PAHs) and dioxins, among others. Exposure to atmospheric PM has been shown to aggravate several autoimmune diseases. Despite strong correlations between exposure to atmospheric PM and worsened autoimmune disease, the mechanisms underlying aggravated disease are largely unknown. The AHR is a ligand activated transcription factor that responds to endogenous and exogenous ligands including toxicants present in PM, such as PAHs and dioxins. A few studies have investigated the effects of atmospheric PM on AHR activation and immune function and demonstrated that atmospheric PM can activate the AHR, change cytokine expression, and alter T cell differentiation. Several studies have found that the AHR modulates the balance between regulatory and effector T cell functions and drives T cell differentiation in vitro and in vivo using murine models of autoimmune disease. However, there are very few studies on the role of AHR in PM-mediated autoimmune disease. The AHR plays a critical role in the balance of effector and regulatory T cells and in autoimmune disease. With increased incidence and prevalence of autoimmune disease occurring concurrently with increases in air pollution, potential mechanisms that drive inflammatory and exacerbated disease need to be elucidated. This review focuses on the AHR as a potential mechanistic target for modulating T cell responses associated with PM-mediated autoimmune disease providing the most up-to-date literature on the role of AHR in autoreactive T cell function and autoimmune disease.
Keywords: T cells; aryl hydrocarbon receptor; atmospheric particulate matter; autoimmune disease; autoimmunity; polycyclic aromatic hydrocarbons.
Figures
Similar articles
-
Ambient urban dust particulate matter reduces pathologic T cells in the CNS and severity of EAE.Environ Res. 2019 Jan;168:178-192. doi: 10.1016/j.envres.2018.09.038. Epub 2018 Oct 1. Environ Res. 2019. PMID: 30316103 Free PMC article.
-
Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro.PLoS One. 2018 Dec 21;13(12):e0209690. doi: 10.1371/journal.pone.0209690. eCollection 2018. PLoS One. 2018. PMID: 30576387 Free PMC article.
-
Real-world PM extracts differentially enhance Th17 differentiation and activate the aryl hydrocarbon receptor (AHR).Toxicology. 2019 Feb 15;414:14-26. doi: 10.1016/j.tox.2019.01.002. Epub 2019 Jan 3. Toxicology. 2019. PMID: 30611761 Free PMC article.
-
The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses.Redox Biol. 2020 Jul;34:101530. doi: 10.1016/j.redox.2020.101530. Epub 2020 Apr 18. Redox Biol. 2020. PMID: 32354640 Free PMC article. Review.
-
Lung cancer associated with combustion particles and fine particulate matter (PM2.5) - The roles of polycyclic aromatic hydrocarbons (PAHs) and the aryl hydrocarbon receptor (AhR).Biochem Pharmacol. 2023 Oct;216:115801. doi: 10.1016/j.bcp.2023.115801. Epub 2023 Sep 9. Biochem Pharmacol. 2023. PMID: 37696458 Free PMC article. Review.
Cited by
-
Autoimmune Diseases Following Environmental Disasters: A Narrative Review of the Literature.Healthcare (Basel). 2024 Sep 4;12(17):1767. doi: 10.3390/healthcare12171767. Healthcare (Basel). 2024. PMID: 39273791 Free PMC article. Review.
-
Environmental exposure and the role of AhR in the tumor microenvironment of breast cancer.Front Pharmacol. 2022 Dec 15;13:1095289. doi: 10.3389/fphar.2022.1095289. eCollection 2022. Front Pharmacol. 2022. PMID: 36588678 Free PMC article. Review.
-
The Role of Aryl Hydrocarbon Receptor in Bone Biology.Int J Tryptophan Res. 2024 May 15;17:11786469241246674. doi: 10.1177/11786469241246674. eCollection 2024. Int J Tryptophan Res. 2024. PMID: 38757095 Free PMC article. Review.
-
Mutated IL-32θ (A94V) inhibits COX2, GM-CSF and CYP1A1 through AhR/ARNT and MAPKs/NF-κB/AP-1 in keratinocytes exposed to PM10.Sci Rep. 2025 Jan 15;15(1):1994. doi: 10.1038/s41598-024-83159-z. Sci Rep. 2025. PMID: 39814789 Free PMC article.
-
Immunotoxicity of Xenobiotics in Fish: A Role for the Aryl Hydrocarbon Receptor (AhR)?Int J Mol Sci. 2021 Aug 31;22(17):9460. doi: 10.3390/ijms22179460. Int J Mol Sci. 2021. PMID: 34502366 Free PMC article. Review.
References
-
- Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, et al. . Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. (2012) 39:259–71. 10.1016/j.jaut.2012.05.002 - DOI - PMC - PubMed
-
- Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. (2015) 3:151–5. 10.12691/ijcd-3-4-8 - DOI
-
- NIO Health. Progress in autoimmune disease research. In: The Autoimmune Diseases Coordinating Committee. Bethesda: National Institute of Health; (2005). p. 1–146.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

