Sofosbuvir, velpatasvir and voxilaprevir: a new triple combination for hepatitis C virus treatment. One pill fits all? Is it the end of the road?
- PMID: 30574189
- PMCID: PMC6295690
- DOI: 10.1177/1756284818812358
Sofosbuvir, velpatasvir and voxilaprevir: a new triple combination for hepatitis C virus treatment. One pill fits all? Is it the end of the road?
Abstract
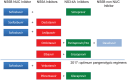
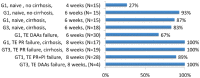
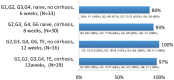
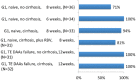
The advent of oral direct-acting antiviral agents (DAAs) has dramatically improved the hepatitis C virus (HCV) treatment landscape in the last 4 years, providing cure rates over 95% with a shorter duration of treatment and a very good safety profile. This has enabled access to treatment in nearly all HCV infected patients. The launch of two pangenotypic fixed dose combinations (FDCs) in 2017 made a new step forward in HCV treatment by slightly increasing efficacy and more importantly allowing the treatment of patients without HCV genotyping, and in some cases without fibrosis assessment. However, retreatment of the few DAA failure patients was still an issue for some HCV genotypes. The launch of the triple regimen FDC, sofosbuvir/velpatasvir/voxilaprevir, solves this issue by providing a cure rate over 96% regardless of HCV genotype. In this review, we describe the current HCV treatment landscape and focus on the development of this triple FDC either in treatment-naïve or treatment-experienced patients with previous failure on a DAA regimen.
Keywords: DAA failure; fixed dose combination; glecaprevir; pibrentasvir; single pill tablet; sofosbuvir; velpatasvir; voxilaprevir.
Conflict of interest statement
Conflict of interest statement: The following are declared: M. Bourlière: advisory board and speaker for: Gilead, AbbVie, MSD, BMS, Janssen, Boehringer-Ingelheim; Paul Castellani: speaker for Gilead, AbbVie, Janssen, and MSD; Valérie Oules: speaker for Gilead, AbbVie, Janssen, MSD; Xavier Adhoute: speaker for Bayer.
Figures
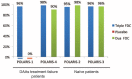
References
-
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2: 161–176. - PubMed
-
- Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, et al. MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. Aids 2017; 31: 1603–1610. - PubMed
-
- Ingiliz P, Martin TC, Rodger A, et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol 2017; 66: 282–287. - PubMed
-
- McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361: 580–593. - PubMed
-
- Shiffman ML, Suter F, Bacon BR, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med 2007; 357: 124–134. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

