Predictive validity of the CriSTAL tool for short-term mortality in older people presenting at Emergency Departments: a prospective study
- PMID: 30574216
- PMCID: PMC6267649
- DOI: 10.1007/s41999-018-0123-6
Predictive validity of the CriSTAL tool for short-term mortality in older people presenting at Emergency Departments: a prospective study
Abstract
Abstract: To determine the validity of the Australian clinical prediction tool Criteria for Screening and Triaging to Appropriate aLternative care (CRISTAL) based on objective clinical criteria to accurately identify risk of death within 3 months of admission among older patients.
Methods: Prospective study of ≥ 65 year-olds presenting at emergency departments in five Australian (Aus) and four Danish (DK) hospitals. Logistic regression analysis was used to model factors for death prediction; Sensitivity, specificity, area under the ROC curve and calibration with bootstrapping techniques were used to describe predictive accuracy.
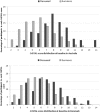
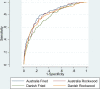
Results: 2493 patients, with median age 78-80 years (DK-Aus). The deceased had significantly higher mean CriSTAL with Australian mean of 8.1 (95% CI 7.7-8.6 vs. 5.8 95% CI 5.6-5.9) and Danish mean 7.1 (95% CI 6.6-7.5 vs. 5.5 95% CI 5.4-5.6). The model with Fried Frailty score was optimal for the Australian cohort but prediction with the Clinical Frailty Scale (CFS) was also good (AUROC 0.825 and 0.81, respectively). Values for the Danish cohort were AUROC 0.764 with Fried and 0.794 using CFS. The most significant independent predictors of short-term death in both cohorts were advanced malignancy, frailty, male gender and advanced age. CriSTAL's accuracy was only modest for in-hospital death prediction in either setting.
Conclusions: The modified CriSTAL tool (with CFS instead of Fried's frailty instrument) has good discriminant power to improve prognostic certainty of short-term mortality for ED physicians in both health systems. This shows promise in enhancing clinician's confidence in initiating earlier end-of-life discussions.
Keywords: Aged; Frail; Prognosis; Prospective studies; Risk assessment; Uncertainty.
Conflict of interest statement
MC and KH developed the risk prediction tool in 2015 and have tested it retrospectively in several hospitals. This might be perceived by readers as non-financial conflict of interest given their knowledge of the subject matter or materials discussed in this manuscript. However, they have no affiliations with or involvement in any organisation or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; or expert testimony or patent-licensing arrangements), that may influence the validity of the study. All other authors certify that they have NO conflict of interest to report. The analyses were led by two co-authors (RMT and FG) who were not involved in the development of the tool, data collection or outcome ascertainment for this validation study.The study was approved in Australia by the hospital management teams and by the South Eastern Sydney Local Health District Ethics Committee [#15/026 HREC/15/POW/55 and data was stored in the UNSW secure server. The need for approval in Denmark was waivered by the Regional Ethics Committee of Southern Denmark, as this project was considered to be a quality improvement initiative without an intervention [42].Verbal information on the study preceded the request for written informed consent which was obtained from each individual if cognitively competent or their surrogate at the time of recruitment. Participants consented to being interviewed, giving access to hospital records during admission, and being contacted for follow-up outcome ascertainment. The original consent remained with the researchers and a copy went to the patient or their family.
Figures

References
-
- Silverman ME, Cochrane DG, Allegra JR, Rothman J. Differential increases in emergency department visits within the geriatric population. Ann Emerg Med. 2004;44(4 Supplement):S71. doi: 10.1016/j.annemergmed.2004.07.234. - DOI
LinkOut - more resources
Full Text Sources
