Crystal structure and Hirshfeld surface analysis of two (E)- N'-(para-substituted benzyl-idene) 4-chloro-benzene-sulfono-hydrazides
- PMID: 30574379
- PMCID: PMC6281117
- DOI: 10.1107/S205698901801592X
Crystal structure and Hirshfeld surface analysis of two (E)- N'-(para-substituted benzyl-idene) 4-chloro-benzene-sulfono-hydrazides
Abstract
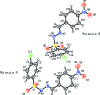
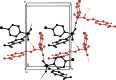
Two (E)-N'-(p-substituted benzyl-idene)-4-chloro-benzene-sulfono-hydrazides, namely, (E)-4-chloro-N'-(4-chloro-benzyl-idene)benzene-sulfono-hydrazide, C13H10Cl2N2O2S, (I), and (E)-4-chloro-N'-(4-nitro-benzyl-idene)benzene-sulfono-hydrazide, C13H10ClN3O4S, (II), have been synthesized, characterized and their crystal structures studied to explore the effect of the nature of substituents on the structural parameters. Compound (II) crystallized with two independent mol-ecules [(IIA) and IIB)] in the asymmetric unit. In both compounds, the configuration around the C=N bond is E. The mol-ecules are twisted at the S atom with C-S-N-N torsion angles of -62.4 (2)° in (I), and -46.8 (2)° and 56.8 (2)° in the mol-ecules A and B of (II). The 4-chloro-phenyl-sulfonyl and 4-substituted benzyl-idene rings form dihedral angles of 81.0 (1)° in (I), 75.9 (1)° in (IIA) and 73.4 (1)° in (IIB). In the crystal of (I), mol-ecules are linked via pairs of N-H⋯O hydrogen bonds, forming inversion dimers with an R 2 2(8) ring motif. The dimers are linked by C-Cl⋯π inter-actions, forming a three-dimensional structure. In the crystal of (II), mol-ecules are linked by C-H⋯π inter-actions and N-H⋯O hydrogen bonds, forming -A-B-A-B- chains along the c-axis direction. The chains are linked via C-H⋯O and C-H⋯π inter-actions, forming layers parallel to the bc plane. Two-dimensional fingerprint plots show that the most significant contacts contributing to the Hirshfeld surface for (I) are H⋯H contacts (26.6%), followed by Cl⋯H/H⋯Cl (21.3%), O⋯H/H⋯O (15.5%) and Cl⋯C/C⋯Cl (10.7%), while for (II) the O⋯H/H⋯O contacts are dominant, with a contribution of 34.8%, followed by H⋯H (15.2%), C⋯H/H⋯C (14.0%) and Cl⋯H/H⋯Cl (10.0%) contacts.
Keywords: Hirshfeld surface analysis; N′-(arylidene)arylsulfonohydrazides; crystal structure; fingerprint plots; graph-set motif; hydrazones; hydrogen bonding.
Figures






References
-
- Hashemi, S. A. (2012). Tetrahedron Lett. 53, 5141–5143.
LinkOut - more resources
Full Text Sources
Research Materials
