Structures of the hydrate and dihydrate forms of the DNA-binding radioprotector methyl-pro-amine
- PMID: 30574398
- PMCID: PMC6281125
- DOI: 10.1107/S2056989018016791
Structures of the hydrate and dihydrate forms of the DNA-binding radioprotector methyl-pro-amine
Abstract
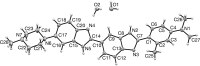
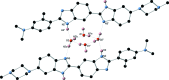
Methyl pro-amine {N,N,3-trimethyl-4-[6-(4-methyl-piperazin-1-yl)-1H,3'H-[2,5'-bibenzo[d]imidazol]-2'-yl]aniline}, C28H35N7O2, crystallized as both a dihydrate, C28H31N7·2H2O, and monohydrate, C28H31N7·H2O, form from water in the presence of β-cyclo-dextrin, in the P21/c and P21/n space groups, respectively. The two structures adopt different conformations and tautomeric forms as a result of the differing crystal packing as dictated by hydrogen-bonding inter-actions. The dihydrate crystallizes as a three-dimensional hydrogen-bonded network, while the monohydrate crystallizes as a two-dimensional hydrogen-bonded network.
Keywords: crystal structure; hydrates; hydrogen bonding; tautomerism.
Figures








References
-
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
-
- Martin, R. F., Broadhurst, S., Reum, M. E., Squire, C. J., Clark, G. R., Lobachevsky, P. N., White, J. M., Clark, C., Sy, D., Spotheim-Maurizot, M. & Kelly, D. P. (2004). Cancer Res. 64, 1067–1070. - PubMed
-
- Palmer, D. C. (2014). CrystalMaker. CrystalMaker Software Ltd, Begbroke, England.
-
- Pjura, P. E., Grzeskowiak, G. & Dickerson, R. E. (1987). J. Mol. Biol. 197, 257–271. - PubMed
LinkOut - more resources
Full Text Sources
