Korean Guideline for the Prevention and Treatment of Glucocorticoid-induced Osteoporosis
- PMID: 30574464
- PMCID: PMC6288607
- DOI: 10.11005/jbm.2018.25.4.195
Korean Guideline for the Prevention and Treatment of Glucocorticoid-induced Osteoporosis
Abstract
Background: To develop guidelines and recommendations to prevent and treat glucocorticoid (GC)-induced osteoporosis (GIOP) in Korea.
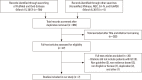
Methods: The Korean Society for Bone and Mineral Research and the Korean College of Rheumatology have developed this guideline based on Guidance for the Development of Clinical Practice Guidelines ver. 1.0 established by the National Evidence-Based Healthcare Collaborating Agency. This guideline was developed by adapting previously published guidelines, and a systematic review and quality assessment were performed.
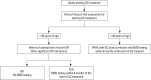
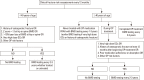
Results: This guideline applies to adults aged ≥19 years who are using or plan to use GCs. It does not include children and adolescents. An initial assessment of fracture risk should be performed within 6 months of initial GC use. Fracture risk should be estimated using the fracture-risk assessment tool (FRAX) after adjustments for GC dose, history of osteoporotic fractures, and bone mineral density (BMD) results. All patients administered with prednisolone or an equivalent medication at a dose ≥2.5 mg/day for ≥3 months are recommended to use adequate calcium and vitamin D during treatment. Patients showing a moderate-to-high fracture risk should be treated with additional medication for osteoporosis. All patients continuing GC therapy should undergo annual BMD testing, vertebral X-ray, and fracture risk assessment using FRAX. When treatment failure is suspected, switching to another drug should be considered.
Conclusions: This guideline is intended to guide clinicians in the prevention and treatment of GIOP.
Keywords: Bisphosphonate; Denosumab; Glucocorticoids; Osteporosis; Teriparatide.
Conflict of interest statement
CONFLICT OF INTEREST: Yoon-Kyung Sung has received financial support for clinical research sponsored by Pfizer within the last 2 years. Dong Ah Park has participated in the development of headache clinical practical guidelines for methodology consultation. The other authors declare no conflict of interest. If a committee member receives research funding from a company, that member does not participate in discussions or votes concerning that company's drug.
Figures

References
-
- Saag KG, Koehnke R, Caldwell JR, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96:115–123. - PubMed
-
- Lane NE, Lukert B. The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin North Am. 1998;27:465–483. - PubMed
-
- Van Staa TP, Laan RF, Barton IP, et al. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. - PubMed
-
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323–328. - PubMed
-
- Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39:253–259. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

