Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma
- PMID: 30575030
- PMCID: PMC6492001
- DOI: 10.1002/path.5224
Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma
Abstract
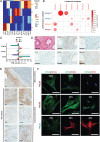
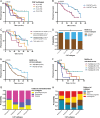
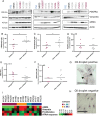
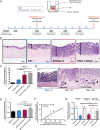
Cancer-associated fibroblasts (CAF) are orchestrators of the pancreatic ductal adenocarcinoma (PDAC) microenvironment. Stromal heterogeneity may explain differential pathophysiological roles of the stroma (pro- versus anti-tumoural) in PDAC. We hypothesised that multiple CAF functional subtypes exist in PDAC, that contribute to stromal heterogeneity through interactions with cancer cells. Using molecular and functional analysis of patient-derived CAF primary cultures, we demonstrated that human PDAC-derived CAFs display a high level of inter- and intra-tumour heterogeneity. We identified at least four subtypes of CAFs based on transcriptomic analysis, and propose a classification for human PDAC-derived CAFs (pCAFassigner). Multiple CAF subtypes co-existed in individual patient samples. The presence of these CAF subtypes in bulk tumours was confirmed using publicly available gene expression profiles, and immunostainings of CAF subtype markers. Each subtype displayed specific phenotypic features (matrix- and immune-related signatures, vimentin and α-smooth muscle actin expression, proliferation rate), and was associated with an assessable prognostic impact. A prolonged exposure of non-tumoural pancreatic stellate cells to conditioned media from cancer cell lines (cancer education experiment) induced a CAF-like phenotype, including loss of capacity to revert to quiescence and an increase in the expression of genes related to CAF subtypes B and C. This classification demonstrates molecular and functional inter- and intra-tumoural heterogeneity of CAFs in human PDAC. Our subtypes overlap with those identified from single-cell analyses in other cancers, and pave the way for the development of therapies targeting specific CAF subpopulations in PDAC. © 2018 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: pancreatic stellate cell; stroma; transcriptomics; tumour microenvironment; tumour-stroma interactions.
© 2018 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures

References
-
- Neesse A, Algul H, Tuveson DA, et al Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 2015; 64: 1476–1484. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

