The Association of Biochemical Hypoglycemia with the Subsequent Risk of a Severe Hypoglycemic Event: Analysis of the DCCT Data Set
- PMID: 30575408
- PMCID: PMC6909677
- DOI: 10.1089/dia.2018.0362
The Association of Biochemical Hypoglycemia with the Subsequent Risk of a Severe Hypoglycemic Event: Analysis of the DCCT Data Set
Abstract
Objective: To evaluate the association of biochemical hypoglycemia with subsequent severe hypoglycemia (SH) events using the Diabetes Control and Complications Trial (DCCT) data set.
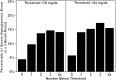
Research design and methods: The frequency of biochemical hypoglycemia (percentage of values <70 and <54 mg/dL [3.9 and 3.0 mmol/L) was assessed using DCCT blood glucose concentrations measured at a central laboratory from seven finger-stick samples (7-point testing: pre- and 90-min postmeals and at bedtime) collected during 1 day every 3 months. SH events required a change in mental status necessitating the involvement of another individual to provide treatment. A Poisson model accounting for repeated measures from each participant was used to assess the association of biochemical hypoglycemia frequency, computed from the 7-point finger-stick data, with the development of SH events.
Results: The risk of SH during a 3-month period was substantially higher (P < 0.001) when there was at least one hypoglycemic blood glucose value in the preceding 7-point profile, with similar results seen for both the 70 mg/dL (rate ratio = 3.0 [95% confidence interval: 2.6-3.3]) and 54 mg/dL (rate ratio = 2.7 [95% confidence interval: 2.4-3.1]) thresholds.
Conclusions: The occurrence of biochemical hypoglycemia <70 or <54 mg/dL is associated with an increased risk of SH. For this reason as well as the deleterious effects of hypoglycemia on glucose counter-regulation and hypoglycemia awareness, cognition, quality of life, and arrhythmias, it is important in diabetes management to avoid hypoglycemic glucose levels as much as possible.
Keywords: Biochemical hypoglycemia; Risk assessment; Severe hypoglycemia; Type 1 diabetes..
Conflict of interest statement
R.W.B., T.D.R., and C.K. have no personal disclosures, but report that their institution has received research funding or study supplies from Abbott, Ascenia, Bigfoot, Dexcom, Roche, Tandem, and consulting fees from Insulet and Lilly. R.M.B. has no personal disclosures, but reports that his employer, the nonprofit Health Partners Institute, contracts for his services, which includes research support, consulting, or scientific advisory board participation for Abbott Diabetes Care, Becton Dickinson, Dexcom, Eli Lilly, Glooko, Hygieia, Johnson & Johnson, Medtronic, Merck, Novo Nordisk, Roche, and Sanofi.
Figures
References
-
- Agiostratidou G, Anhalt H, Ball D, et al. : Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40:1622–1630 - PMC - PubMed
-
- Beyond ACWG: Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care 2018;41:e92–e94 - PubMed
-
- International Hypoglycaemia Study G: Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017;40:155–157 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical