Loss of Phd2 cooperates with BRAFV600E to drive melanomagenesis
- PMID: 30575721
- PMCID: PMC6303344
- DOI: 10.1038/s41467-018-07126-9
Loss of Phd2 cooperates with BRAFV600E to drive melanomagenesis
Erratum in
-
Author Correction: Loss of Phd2 cooperates with BRAFV600E to drive melanomagenesis.Nat Commun. 2019 Mar 11;10(1):1211. doi: 10.1038/s41467-019-09195-w. Nat Commun. 2019. PMID: 30858377 Free PMC article.
Abstract
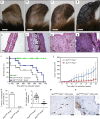
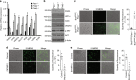
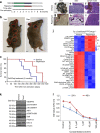
Prolyl hydroxylase domain protein 2 (PHD2) is a well-known master oxygen sensor. However, the role of PHD2 in tumor initiation remains controversial. We find that during the transition of human nevi to melanoma, the expression of PHD2 protein is significantly decreased and lower expression PHD2 in melanoma is associated with worse clinical outcome. Knockdown of PHD2 leads to elevated Akt phosphorylation in human melanocytes. Mice with conditional melanocyte-specific expression of Phd2lox/lox (Tyr::CreER;Phd2lox/lox) fail to develop pigmented lesions. However, deletion of Phd2 in combination with expression of BRafV600E in melanocytes (Tyr::CreER;Phd2lox/lox;BRafCA) leads to the development of melanoma with 100% penetrance and frequent lymph node metastasis. Analysis of tumor tissues using reverse phase protein arrays demonstrates that Phd2 deletion activates the AKT-mTOR-S6 signaling axis in the recovered tumors. These data indicate that PHD2 is capable of suppressing tumor initiation largely mediated through inhibiting of the Akt-mTOR signaling pathway in the melanocyte lineage.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

