Association of Corticosteroid Treatment With Outcomes in Adult Patients With Sepsis: A Systematic Review and Meta-analysis
- PMID: 30575845
- PMCID: PMC6439648
- DOI: 10.1001/jamainternmed.2018.5849
Association of Corticosteroid Treatment With Outcomes in Adult Patients With Sepsis: A Systematic Review and Meta-analysis
Abstract
Importance: Although corticosteroids are widely used for adults with sepsis, both the overall benefit and potential risks remain unclear.
Objective: To conduct a systematic review and meta-analysis of the efficacy and safety of corticosteroids in patients with sepsis.
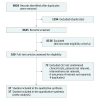
Data sources and study selection: MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception until March 20, 2018, and updated on August 10, 2018. The terms corticosteroids, sepsis, septic shock, hydrocortisone, controlled trials, and randomized controlled trial were searched alone or in combination. Randomized clinical trials (RCTs) were included that compared administration of corticosteroids with placebo or standard supportive care in adults with sepsis.
Data extraction and synthesis: Meta-analyses were conducted using a random-effects model to calculate risk ratios (RRs) and mean differences (MDs) with corresponding 95% CIs. Two independent reviewers completed citation screening, data abstraction, and risk assessment.
Main outcomes and measures: Twenty-eight-day mortality.
Results: This meta-analysis included 37 RCTs (N = 9564 patients). Eleven trials were rated as low risk of bias. Corticosteroid use was associated with reduced 28-day mortality (RR, 0.90; 95% CI, 0.82-0.98; I2 = 27%) and intensive care unit (ICU) mortality (RR, 0.85; 95% CI, 0.77-0.94; I2 = 0%) and in-hospital mortality (RR, 0.88; 95% CI, 0.79-0.99; I2 = 38%). Corticosteroids were significantly associated with increased shock reversal at day 7 (MD, 1.95; 95% CI, 0.80-3.11) and vasopressor-free days (MD, 1.95; 95% CI, 0.80-3.11) and with ICU length of stay (MD, -1.16; 95% CI, -2.12 to -0.20), the sequential organ failure assessment score at day 7 (MD, -1.38; 95% CI, -1.87 to -0.89), and time to resolution of shock (MD, -1.35; 95% CI, -1.78 to -0.91). However, corticosteroid use was associated with increased risk of hyperglycemia (RR, 1.19; 95% CI, 1.08-1.30) and hypernatremia (RR, 1.57; 95% CI, 1.24-1.99).
Conclusions and relevance: The findings suggest that administration of corticosteroids is associated with reduced 28-day mortality compared with placebo use or standard supportive care. More research is needed to associate personalized medicine with the corticosteroid treatment to select suitable patients who are more likely to show a benefit.
Conflict of interest statement
Figures

Comment in
-
Future Directions for Corticosteroids in Treatment of Sepsis.JAMA Intern Med. 2019 Jun 1;179(6):845. doi: 10.1001/jamainternmed.2019.0859. JAMA Intern Med. 2019. PMID: 31157838 Free PMC article. No abstract available.
-
Future Directions for Corticosteroids in Treatment of Sepsis-Reply.JAMA Intern Med. 2019 Jun 1;179(6):845. doi: 10.1001/jamainternmed.2019.0856. JAMA Intern Med. 2019. PMID: 31157848 No abstract available.
References
-
- Shankar-Hari M, Phillips GS, Levy ML, et al. ; Sepsis Definitions Task Force . Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):775-787. doi: 10.1001/jama.2016.0289 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

