Human CYP2A6, CYP2B6, AND CYP2E1 Atropselectively Metabolize Polychlorinated Biphenyls to Hydroxylated Metabolites
- PMID: 30576102
- PMCID: PMC6380921
- DOI: 10.1021/acs.est.8b05250
Human CYP2A6, CYP2B6, AND CYP2E1 Atropselectively Metabolize Polychlorinated Biphenyls to Hydroxylated Metabolites
Abstract
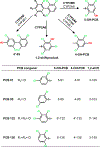
Exposure to chiral polychlorinated biphenyls (PCBs) has been associated with neurodevelopmental disorders. Their hydroxylated metabolites (OH-PCBs) are also potentially toxic to the developing human brain; however, the formation of OH-PCBs by human cytochrome P450 (P450) isoforms is poorly investigated. To address this knowledge gap, we investigated the atropselective biotransformation of 2,2',3,4',6-pentachlorobiphenyl (PCB 91), 2,2',3,5',6-pentachlorobiphenyl (PCB 95), 2,2',3,3',4,6'-hexachlorobiphenyl (PCB 132), and 2,2',3,3',6,6'-hexachlorobiphenyl (PCB 136) by different human P450 isoforms. In silico predictions with ADMET Predictor and MetaDrug software suggested a role of CYP1A2, CYP2A6, CYP2B6, CYP2E1, and CYP3A4 in the metabolism of chiral PCBs. Metabolism studies with recombinant human enzymes demonstrated that CYP2A6 and CYP2B6 oxidized PCB 91 and PCB 132 in the meta position and that CYP2A6 oxidized PCB 95 and PCB 136 in the para position. CYP2B6 played only a minor role in the metabolism of PCB 95 and PCB 136 and formed meta-hydroxylated metabolites. Traces of para-hydroxylated PCB metabolites were detected in incubations with CYP2E1. No hydroxylated metabolites were present in incubations with CYP1A2 or CYP3A4. Atropselective analysis revealed P450 isoform-dependent and congener-specific atropselective enrichment of OH-PCB metabolites. These findings suggest that CYP2A6 and CYP2B6 play an important role in the oxidation of neurotoxic PCBs to chiral OH-PCBs in humans.
Figures




Similar articles
-
Human Liver Microsomes Atropselectively Metabolize 2,2',3,4',6-Pentachlorobiphenyl (PCB 91) to a 1,2-Shift Product as the Major Metabolite.Environ Sci Technol. 2018 May 15;52(10):6000-6008. doi: 10.1021/acs.est.8b00612. Epub 2018 Apr 27. Environ Sci Technol. 2018. PMID: 29659268 Free PMC article.
-
Atropselective Disposition of 2,2',3,4',6-Pentachlorobiphenyl (PCB 91) and Identification of Its Metabolites in Mice with Liver-Specific Deletion of Cytochrome P450 Reductase.Chem Res Toxicol. 2020 Jun 15;33(6):1328-1338. doi: 10.1021/acs.chemrestox.9b00255. Epub 2019 Aug 26. Chem Res Toxicol. 2020. PMID: 31403789 Free PMC article.
-
Metabolic activation of WHO-congeners PCB28, 52, and 101 by human CYP2A6: evidence from in vitro and in vivo experiments.Arch Toxicol. 2024 Nov;98(11):3739-3753. doi: 10.1007/s00204-024-03836-w. Epub 2024 Aug 13. Arch Toxicol. 2024. PMID: 39136732 Free PMC article.
-
Chiral polychlorinated biphenyls: absorption, metabolism and excretion--a review.Environ Sci Pollut Res Int. 2016 Feb;23(3):2042-57. doi: 10.1007/s11356-015-4150-2. Epub 2015 Feb 6. Environ Sci Pollut Res Int. 2016. PMID: 25651810 Free PMC article. Review.
-
Complex roles for sulfation in the toxicities of polychlorinated biphenyls.Crit Rev Toxicol. 2024 Feb;54(2):92-122. doi: 10.1080/10408444.2024.2311270. Epub 2024 Feb 16. Crit Rev Toxicol. 2024. PMID: 38363552 Free PMC article. Review.
Cited by
-
Placenta and fetal brain share a neurodevelopmental disorder DNA methylation profile in a mouse model of prenatal PCB exposure.Cell Rep. 2022 Mar 1;38(9):110442. doi: 10.1016/j.celrep.2022.110442. Cell Rep. 2022. PMID: 35235788 Free PMC article.
-
Phenylalanine Residues in the Active Site of CYP2E1 Participate in Determining the Binding Orientation and Metabolism-Dependent Genotoxicity of Aromatic Compounds.Toxics. 2023 May 31;11(6):495. doi: 10.3390/toxics11060495. Toxics. 2023. PMID: 37368596 Free PMC article.
-
Gene×environment interactions in autism spectrum disorders.Curr Top Dev Biol. 2023;152:221-284. doi: 10.1016/bs.ctdb.2022.11.001. Epub 2022 Dec 19. Curr Top Dev Biol. 2023. PMID: 36707213 Free PMC article. Review.
-
Atropselective Partitioning of Polychlorinated Biphenyls in a HepG2 Cell Culture System: Experimental and Modeling Results.Environ Sci Technol. 2020 Nov 3;54(21):13817-13827. doi: 10.1021/acs.est.0c02508. Epub 2020 Oct 15. Environ Sci Technol. 2020. PMID: 33059451 Free PMC article.
-
Characterization of the Metabolic Pathways of 4-Chlorobiphenyl (PCB3) in HepG2 Cells Using the Metabolite Profiles of Its Hydroxylated Metabolites.Environ Sci Technol. 2021 Jul 6;55(13):9052-9062. doi: 10.1021/acs.est.1c01076. Epub 2021 Jun 14. Environ Sci Technol. 2021. PMID: 34125531 Free PMC article.
References
-
- EPA Polychlorinated Biphenyls (PCBs) https://www.epa.gov/pcbs (accessed 12/03/2018),
-
- ATSDR Toxicological profile for polychlorinated biphenyls (PCBs) U.S. Dept. Health Services, Public Health Service; 2000. - PubMed
-
- EPA EPA Bans PCB Manufacture; Phases Out Uses https://archive.epa.gov/epa/aboutepa/epa-bans-pcb-manufacture-phases-out... (accessed 2/13/2018),
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

