Photoinitiated Three-Component α-Perfluoroalkyl-β-heteroarylation of Unactivated Alkenes via Electron Catalysis
- PMID: 30576162
- PMCID: PMC6326532
- DOI: 10.1021/acs.orglett.8b03849
Photoinitiated Three-Component α-Perfluoroalkyl-β-heteroarylation of Unactivated Alkenes via Electron Catalysis
Abstract
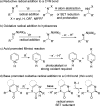
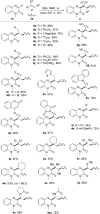
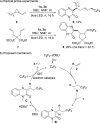
A visible-light-initiated α-perfluoroalkyl-β-heteroarylation of various alkenes with perfluoroalkyl iodides and quinoxalin-2(1 H)-ones is presented. This three-component radical cascade reaction allows an efficient synthesis of a range of perfluoroalkyl containing quinoxalin-2(1 H)-one derivatives in moderate to excellent yields under mild conditions. Reactions proceed via acidic aminyl radicals that are readily deprotonated to give the corresponding radical anions able to sustain the radical chain as single electron transfer reducing reagents. Hence, the overall cascade classifies as an electron-catalyzed process.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

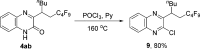
Similar articles
-
Iodoperfluoroalkylation of unactivated alkenes via pyridine-boryl radical initiated atom-transfer radical addition.Org Biomol Chem. 2022 Apr 6;20(14):2857-2862. doi: 10.1039/d2ob00453d. Org Biomol Chem. 2022. PMID: 35297935
-
When Light Meets Nitrogen-Centered Radicals: From Reagents to Catalysts.Acc Chem Res. 2020 May 19;53(5):1066-1083. doi: 10.1021/acs.accounts.0c00090. Epub 2020 Apr 14. Acc Chem Res. 2020. PMID: 32286794
-
α-Perfluoroalkyl-β-alkynylation of alkenes via radical alkynyl migration.Chem Sci. 2017 Oct 1;8(10):6888-6892. doi: 10.1039/c7sc02175e. Epub 2017 Aug 4. Chem Sci. 2017. PMID: 29147514 Free PMC article.
-
The Prowess of Photogenerated Amine Radical Cations in Cascade Reactions: From Carbocycles to Heterocycles.Acc Chem Res. 2016 Sep 20;49(9):1957-68. doi: 10.1021/acs.accounts.6b00263. Epub 2016 Aug 18. Acc Chem Res. 2016. PMID: 27536956
-
Difunctionalization of Alkenes and Alkynes via Intermolecular Radical and Nucleophilic Additions.Molecules. 2020 Dec 28;26(1):105. doi: 10.3390/molecules26010105. Molecules. 2020. PMID: 33379397 Free PMC article. Review.
Cited by
-
Alkyl Radical Addition to Aliphatic and Aromatic N-Acylhydrazones Using an Organic Photoredox Catalyst.Org Lett. 2019 Oct 18;21(20):8290-8294. doi: 10.1021/acs.orglett.9b03053. Epub 2019 Sep 27. Org Lett. 2019. PMID: 31560554 Free PMC article.
-
Photoinitiated decarboxylative C3-difluoroarylmethylation of quinoxalin-2(1H)-ones with potassium 2,2-difluoro-2-arylacetates in water.RSC Adv. 2020 Mar 12;10(18):10559-10568. doi: 10.1039/d0ra02059a. eCollection 2020 Mar 11. RSC Adv. 2020. PMID: 35492892 Free PMC article.
-
Radical chain monoalkylation of pyridines.Chem Sci. 2021 Nov 3;12(46):15362-15373. doi: 10.1039/d1sc02748d. eCollection 2021 Dec 1. Chem Sci. 2021. PMID: 34976357 Free PMC article.
-
Recent Advances in Visible Light-Induced C-H Functionalization of Imidazo[1,2-a]pyridines.Molecules. 2025 Jan 30;30(3):607. doi: 10.3390/molecules30030607. Molecules. 2025. PMID: 39942710 Free PMC article. Review.
-
Metal-Free Direct C-H Functionalization of Quinoxalin-2(1H)-Ones to Produce 3-Vinylated Quinoxalin-2(1H)-Ones in the Presence of Alkenes.Front Chem. 2021 Apr 30;9:672051. doi: 10.3389/fchem.2021.672051. eCollection 2021. Front Chem. 2021. PMID: 33996765 Free PMC article.
References
-
-
For reviews and book chapters:
- Curran D. P.; Porter N. A.; Giese B.. Stereochemistry of Radical Reactions: Concepts, Guidelines and Synthetic Applications; Wiley-VCH Verlag: Weinheim, Germany, 1996.
- Radicals in Organic Synthesis; Sibi M. P.; Renaud P., Eds.; Wiley-VCH: New York, 2001.
- Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu C., Studer A., Eds.; John Wiley and Sons: Chichester, U.K., 2012.
- Bar G.; Parsons A. F. Chem. Soc. Rev. 2003, 32, 251.10.1039/b111414j. - DOI - PubMed
-
-
-
For reviews of radical additions to C = N bonds:
- Miyabe H. Synlett 2012, 23, 1709.10.1055/s-0031-1290378. - DOI
- Friestad G. K. Tetrahedron 2001, 57, 5461.10.1016/S0040-4020(01)00384-2. - DOI
- Miyabe H.; Ueda M.; Naito T. Synlett 2004, 1140.10.1055/s-2004-822889. - DOI
- Friestad G. K. Eur. J. Org. Chem. 2005, 2005, 3157.10.1002/ejoc.200500232. - DOI
- Friestad G. K.; Mathies A. K. Tetrahedron 2007, 63, 2541.10.1016/j.tet.2006.11.076. - DOI - PMC - PubMed
- Yamada K.; Tomioka K. Chem. Rev. 2008, 108, 2874.10.1021/cr078370u. - DOI - PubMed
-
-
-
Selected examples:
- Friestad G. K.; Shen Y.; Ruggles E. Angew. Chem., Int. Ed. 2003, 42, 5061.(Angew. Chem. 2003, 115, 5215)10.1002/anie.200352104. - DOI - PubMed
- Rono L. J.; Yayla H. G.; Wang D. Y.; Armstrong M. F.; Knowles R. R. J. Am. Chem. Soc. 2013, 135, 17735.10.1021/ja4100595. - DOI - PubMed
- Vo C.-V. T.; Luescher M. U.; Bode J. W. Nat. Chem. 2014, 6, 310.10.1038/nchem.1878. - DOI - PubMed
- Slater K.; Friestad G. K. J. Org. Chem. 2015, 80, 6432.10.1021/acs.joc.5b00863. - DOI - PubMed
-
-
-
For rewiews of C(sp2)–H functionalization of hydrazones:
- Xu P.; Li W.; Xie J.; Zhu C. Acc. Chem. Res. 2018, 51, 484.10.1021/acs.accounts.7b00565. - DOI - PubMed
- Xu X.; Zhang J.; Xia H.; Wu J. Org. Biomol. Chem. 2018, 16, 1227.10.1039/C8OB00056E. - DOI - PubMed
- Guo R.; Chen J. RSC Adv. 2018, 8, 17110.10.1039/C8RA02533A. - DOI - PMC - PubMed
- Prieto A.; Bouyssi D.; Monteiro N. Eur. J. Org. Chem. 2018, 2018, 2378.10.1002/ejoc.201701600. - DOI
-
-
-
For selected examples of C(sp2)–H functionalization of hydrazones:
- Xu P.; Wang G.; Zhu Y.; Li W.; Cheng Y.; Li S.; Zhu C. Angew. Chem., Int. Ed. 2016, 55, 2939.(Angew. Chem.2016, 128, 2992)10.1002/anie.201508698. - DOI - PubMed
- Xie J.; Zhang T.; Chen F.; Mehrkens N.; Rominger F.; Rudolph M.; Hashmi A. S. K. Angew. Chem., Int. Ed. 2016, 55, 2934.(Angew. Chem.2016, 128, 2987)10.1002/anie.201508622. - DOI - PubMed
- Janhsen B.; Studer A. J. Org. Chem. 2017, 82, 11703.10.1021/acs.joc.7b00934. - DOI - PMC - PubMed
- Zhang M.; Duan Y.; Li W.; Xu P.; Cheng J.; Yu S.; Zhu C. Org. Lett. 2016, 18, 5356.10.1021/acs.orglett.6b02711. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

