Photoinitiated Three-Component α-Perfluoroalkyl-β-heteroarylation of Unactivated Alkenes via Electron Catalysis
- PMID: 30576162
- PMCID: PMC6326532
- DOI: 10.1021/acs.orglett.8b03849
Photoinitiated Three-Component α-Perfluoroalkyl-β-heteroarylation of Unactivated Alkenes via Electron Catalysis
Abstract
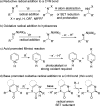
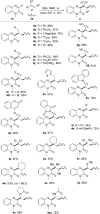
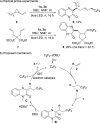
A visible-light-initiated α-perfluoroalkyl-β-heteroarylation of various alkenes with perfluoroalkyl iodides and quinoxalin-2(1 H)-ones is presented. This three-component radical cascade reaction allows an efficient synthesis of a range of perfluoroalkyl containing quinoxalin-2(1 H)-one derivatives in moderate to excellent yields under mild conditions. Reactions proceed via acidic aminyl radicals that are readily deprotonated to give the corresponding radical anions able to sustain the radical chain as single electron transfer reducing reagents. Hence, the overall cascade classifies as an electron-catalyzed process.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

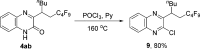
References
-
-
For reviews and book chapters:
- Curran D. P.; Porter N. A.; Giese B.. Stereochemistry of Radical Reactions: Concepts, Guidelines and Synthetic Applications; Wiley-VCH Verlag: Weinheim, Germany, 1996.
- Radicals in Organic Synthesis; Sibi M. P.; Renaud P., Eds.; Wiley-VCH: New York, 2001.
- Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu C., Studer A., Eds.; John Wiley and Sons: Chichester, U.K., 2012.
- Bar G.; Parsons A. F. Chem. Soc. Rev. 2003, 32, 251. 10.1039/b111414j. - DOI - PubMed
-
-
-
For reviews of radical additions to C = N bonds:
- Miyabe H. Synlett 2012, 23, 1709. 10.1055/s-0031-1290378. - DOI
- Friestad G. K. Tetrahedron 2001, 57, 5461. 10.1016/S0040-4020(01)00384-2. - DOI
- Miyabe H.; Ueda M.; Naito T. Synlett 2004, 1140. 10.1055/s-2004-822889. - DOI
- Friestad G. K. Eur. J. Org. Chem. 2005, 2005, 3157. 10.1002/ejoc.200500232. - DOI
- Friestad G. K.; Mathies A. K. Tetrahedron 2007, 63, 2541. 10.1016/j.tet.2006.11.076. - DOI - PMC - PubMed
- Yamada K.; Tomioka K. Chem. Rev. 2008, 108, 2874. 10.1021/cr078370u. - DOI - PubMed
-
-
-
Selected examples:
- Friestad G. K.; Shen Y.; Ruggles E. Angew. Chem., Int. Ed. 2003, 42, 5061.(Angew. Chem. 2003, 115, 5215) 10.1002/anie.200352104. - DOI - PubMed
- Rono L. J.; Yayla H. G.; Wang D. Y.; Armstrong M. F.; Knowles R. R. J. Am. Chem. Soc. 2013, 135, 17735. 10.1021/ja4100595. - DOI - PubMed
- Vo C.-V. T.; Luescher M. U.; Bode J. W. Nat. Chem. 2014, 6, 310. 10.1038/nchem.1878. - DOI - PubMed
- Slater K.; Friestad G. K. J. Org. Chem. 2015, 80, 6432. 10.1021/acs.joc.5b00863. - DOI - PubMed
-
-
-
For rewiews of C(sp2)–H functionalization of hydrazones:
- Xu P.; Li W.; Xie J.; Zhu C. Acc. Chem. Res. 2018, 51, 484. 10.1021/acs.accounts.7b00565. - DOI - PubMed
- Xu X.; Zhang J.; Xia H.; Wu J. Org. Biomol. Chem. 2018, 16, 1227. 10.1039/C8OB00056E. - DOI - PubMed
- Guo R.; Chen J. RSC Adv. 2018, 8, 17110. 10.1039/C8RA02533A. - DOI - PMC - PubMed
- Prieto A.; Bouyssi D.; Monteiro N. Eur. J. Org. Chem. 2018, 2018, 2378. 10.1002/ejoc.201701600. - DOI
-
-
-
For selected examples of C(sp2)–H functionalization of hydrazones:
- Xu P.; Wang G.; Zhu Y.; Li W.; Cheng Y.; Li S.; Zhu C. Angew. Chem., Int. Ed. 2016, 55, 2939.(Angew. Chem.2016, 128, 2992) 10.1002/anie.201508698. - DOI - PubMed
- Xie J.; Zhang T.; Chen F.; Mehrkens N.; Rominger F.; Rudolph M.; Hashmi A. S. K. Angew. Chem., Int. Ed. 2016, 55, 2934.(Angew. Chem.2016, 128, 2987) 10.1002/anie.201508622. - DOI - PubMed
- Janhsen B.; Studer A. J. Org. Chem. 2017, 82, 11703. 10.1021/acs.joc.7b00934. - DOI - PMC - PubMed
- Zhang M.; Duan Y.; Li W.; Xu P.; Cheng J.; Yu S.; Zhu C. Org. Lett. 2016, 18, 5356. 10.1021/acs.orglett.6b02711. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

