Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments
- PMID: 30576244
- PMCID: PMC6415716
- DOI: 10.1152/ajpendo.00438.2018
Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments
Abstract
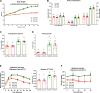
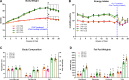
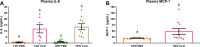
Depletion of macrophages is thought to be a therapeutic option for obesity-induced inflammation and metabolic dysfunction. However, whether the therapeutic effect is a direct result of reduced macrophage-derived inflammation or secondary to decreases in fat mass is controversial, as macrophage depletion has been shown to disrupt energy homeostasis. This study was designed to determine if macrophage depletion via clodronate-liposome (CLD) treatment could serve as an effective intervention to reduce obesity-driven inflammatory and metabolic impairments independent of changes in energy intake. After 16 wk on a high-fat diet (HFD) or the AIN-76A control (low-fat) diet (LFD) ( n = 30/diet treatment), male C57BL/6J mice were assigned to a CLD- or PBS-liposome treatment ( n = 15/group) for 4 wk. Liposomes were administered biweekly via intraperitoneal injections (8 administrations in total). PBS-liposome-treated groups were pair-fed to their CLD-treated dietary counterparts. Metabolic function was assessed before and after liposome treatment. Adipose tissue, as well as the liver, was investigated for macrophage infiltration and the presence of inflammatory mediators. Additionally, a complete blood count was performed. CLD treatment reduced energy intake. When controlling for energy intake, CLD treatment was unable to regress metabolic dysfunction or nonalcoholic fatty liver disease and impaired adipose tissue insulin action. Moreover, repeated CLD treatment induced neutrophilia and anemia, increased adipose tissue mRNA expression of the proinflammatory cytokines IL-6 and IL-1β, and augmented circulating IL-6 and monocyte chemoattractant protein-1 concentrations ( P < 0.05). This study suggests that repeated intraperitoneal administration of CLD to deplete macrophages attenuates obesity by limiting energy intake. Moreover, after controlling for the benefits of weight loss, the accompanying detrimental side effects limit regular CLD treatment as an effective therapeutic strategy.
Keywords: clodronate; inflammation; macrophage; metabolism; obesity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







References
-
- Bader JE, Enos RT, Velázquez KT, Carson MS, Nagarkatti M, Nagarkatti PS, Chatzistamou I, Davis JM, Carson JA, Robinson CM, Murphy EA. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am J Physiol Gastrointest Liver Physiol 314: G22–G31, 2018. doi:10.1152/ajpgi.00229.2017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

