Physiological relevance of epithelial geometry: New insights into the standing gradient model and the role of LI cadherin
- PMID: 30576326
- PMCID: PMC6303100
- DOI: 10.1371/journal.pone.0208791
Physiological relevance of epithelial geometry: New insights into the standing gradient model and the role of LI cadherin
Abstract
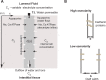
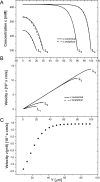
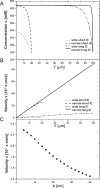
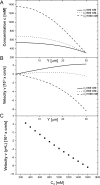
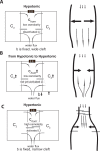
We introduce a mathematical model of an absorbing leaky epithelium to reconsider the problem formulated by Diamond and Bossert in 1967: whether "… some distinctive physiological properties of epithelia might arise as geometrical consequences of epithelial ultrastructure". A standing gradient model of the intercellular cleft (IC) is presented that includes tight junctions (TJ) and ion channels uniformly distributed along the whole cleft. This nonlinear system has an intrinsic homogeneous concentration and the spatial scale necessary to establish it along the cleft. These parameters have not been elucidated so far. We further provide non-perturbative analytical approximations for a broad range of parameters. We found that narrowing of the IC increases ion concentration dramatically and can therefore prevent outflow through tight junctions (TJs) and the lateral membrane, as long as extremely high luminal osmolarities are not reached. Our model predicts that the system is to some extent self-regulating and thereby prevents fluxes into the lumen. Recent experimental evidence has shown that liver-intestine (LI) cadherin can control the up/down flux in intestines via regulation of the cleft width. This finding is in full agreement with predictions of our model. We suggest that LI-cadherin may increase water transport through epithelia via sequential narrowing of the cleft, starting from the highest concentration area at the beginning of the cleft and triggering a propagating squeezing motion.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The function of 7D-cadherins: a mathematical model predicts physiological importance for water transport through simple epithelia.Theor Biol Med Model. 2011 Jun 10;8:18. doi: 10.1186/1742-4682-8-18. Theor Biol Med Model. 2011. PMID: 21663598 Free PMC article.
-
Water transport through the intestinal epithelial barrier under different osmotic conditions is dependent on LI-cadherin trans-interaction.Tissue Barriers. 2017 Apr 3;5(2):e1285390. doi: 10.1080/21688370.2017.1285390. Epub 2017 Jan 24. Tissue Barriers. 2017. PMID: 28452574 Free PMC article.
-
Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types.BMC Biol. 2018 Feb 1;16(1):19. doi: 10.1186/s12915-018-0481-z. BMC Biol. 2018. PMID: 29391007 Free PMC article.
-
Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens.Toxins (Basel). 2017 Feb 10;9(2):60. doi: 10.3390/toxins9020060. Toxins (Basel). 2017. PMID: 28208612 Free PMC article. Review.
-
Models for coupling of salt and water transport; Proximal tubular reabsorption in Necturus kidney.J Gen Physiol. 1975 Dec;66(6):671-733. doi: 10.1085/jgp.66.6.671. J Gen Physiol. 1975. PMID: 1104761 Free PMC article. Review.
Cited by
-
Angulin-1 (LSR) Affects Paracellular Water Transport, However Only in Tight Epithelial Cells.Int J Mol Sci. 2021 Jul 22;22(15):7827. doi: 10.3390/ijms22157827. Int J Mol Sci. 2021. PMID: 34360593 Free PMC article.
References
-
- Parsons DS, Wingate DL. The effect of osmotic gradients on fluid transfer across rat 600 intestine in vitro. Biochim Biophys Acta. 1961; 46: 170–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

