Real-world effectiveness and safety of sofosbuvir and ledipasvir with or without ribavirin for patients with hepatitis C virus genotype 1 infection in Taiwan
- PMID: 30576344
- PMCID: PMC6303025
- DOI: 10.1371/journal.pone.0209299
Real-world effectiveness and safety of sofosbuvir and ledipasvir with or without ribavirin for patients with hepatitis C virus genotype 1 infection in Taiwan
Abstract
Background: The real-world data for the effectiveness and safety of sofosbuvir/ledipasvir (SOF/LDV) with or without ribavirin (RBV) in patients with hepatitis C virus genotype 1 (HCV-1) infection remain limited in Taiwan.
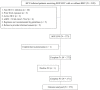
Methods: A total of 273 chronic HCV-1 patients receiving 8, 12, or 24 weeks of SOF/LDV with or without RBV were enrolled. The sustained virologic response rate at week 12 off-therapy (SVR12) by evaluable population (EP) and per-protocol population (PP) were assessed for effectiveness. The treatment discontinuation rate due to adverse events (AEs) and serious AE rate were assessed for safety. Baseline patient characteristics and on-treatment HCV viral kinetics associated with SVR12 were analyzed.
Results: The SVR12 rates by EP and PP analyses were 96.7% (95% confidence interval [CI]: 93.9%-98.3%) and 97.5% (95% CI: 94.8%-98.8%), respectively. The rates of treatment discontinuation due to AE and serious AE were 0.4% and 4.4%, respectively. Seven patients with true virologic failure were relapsers. In 2 patients who were lost-to follow-up, one expired at treatment week 3 due to pneumonia which was considered not related to treatment, and one declined follow-up at off-therapy week 4. The SVR12 rates were comparable in terms of baseline patient characteristics and viral decline at week 4 of treatment.
Conclusions: SOF/LDV with or without RBV for 8-24 weeks is well tolerated and achieves a high SVR12 rate in patients with HCV-1 infection in Taiwan.
Conflict of interest statement
Chen-Hua Liu: advisory board for Abbvie, Gilead Science, Merck Sharp & Dohme; speaker’s bureau for Abbott, Abbvie, Gilead Science, Merck Sharp & Dohme; research grant from Abbvie, Gilead Science, Merck Sharp & Dohme. Jia- Horng Kao: advisory board for Abbott, Abbvie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Roche; speaker’s bureau for Abbott, Abbvie, Bayer, Bristol-Myers Squibb, Gilead Science, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Roche. Ding-Shinn Chen: advisory board for Abbvie, Gilead Science, GlaxoSmithKline, Merck Sharp & Dohme, Novartis. Pei-Jer Chen: advisory board for Abbvie, Novartis, Roche. All other authors declare no competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
References
-
- Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, et al. ; R.E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206: 469–477. 10.1093/infdis/jis385 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous