Heterochronic reproductive developmental processes between diploid and tetraploid cytotypes of Paspalum rufum
- PMID: 30576402
- PMCID: PMC6526369
- DOI: 10.1093/aob/mcy228
Heterochronic reproductive developmental processes between diploid and tetraploid cytotypes of Paspalum rufum
Abstract
Background and aims: Apomixis is an asexual reproductive mode via seeds that generate maternal clonal progenies. Although apomixis in grasses is mainly expressed at the polyploid level, some natural diploid genotypes of Paspalum rufum produce aposporous embryo sacs in relatively high proportions and are even able to complete apomixis under specific conditions. However, despite the potential for apomixis, sexuality prevails in diploids, and apomixis expression is repressed for an as yet undetermind reason. Apomixis is thought to derive from a deregulation of one or a few components of the sexual pathway that could be triggered by polyploidy and/or hybridization. The objectives of this work were to characterize and compare the reproductive development and the timing of apospory initial (AI) emergence between diploid genotypes with potential for apomixis and facultative apomictic tetraploid cytotypes of P. rufum.
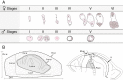
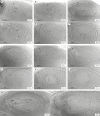
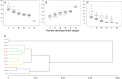
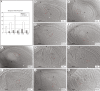
Methods: Reproductive characterization was performed by cytoembryological observations of cleared ovaries and anthers during all reproductive development steps and by quantitative evaluation of the ovule growth parameters.
Key results: Cytoembryological observations showed that in diploids, both female and male reproductive development is equally synchronized, but in tetraploids, megasporogenesis and early megagametogenesis are delayed with respect to microsporogenesis and early microgametogenesis. This delay was also seen when ovary growth was taken as a reference parameter. The analysis of the onset of AIs revealed that they emerge during different developmental periods depending on the ploidy level. In diploids, the AIs appeared along with the tetrad (or triad) of female meiocytes, but in tetraploids they appeared earlier, at the time of the megaspore mother cell. In both cytotypes, AIs can be seen even during megagametogenesis.
Conclusions: Overall observations reveal that female sexual reproductive development is delayed in tetraploids as compared with diploid genotypes, mainly at meiosis. In tetraploids, AIs appear at earlier sexual developmental stages than in diploids, and they accumulate up to the end of megasporogenesis. The longer extension of megasporogenesis in tetraploids could favour AI emergence and also apomixis success.
Keywords: Paspalum rufum; Apomixis; apospory; apospory initial; heterochrony; polyploidy; reproductive development; sexuality.
© The Author(s) 2018. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Acuña CA, Martínez EJ, Quarin CL. 2005. Sexual diploid and apomictic tetraploid races in Thrasya petrosa (Gramineae). Australian Journal of Botany 53: 479–484.
-
- Acuña CA, Blount AR, Quesenberry KH, Kenworthy KE, Hanna WW. 2009. Bahiagrass tetraploid germplasm: reproductive and agronomic characterization of segregating progeny. Crop Science 49: 581.
-
- Acuña C, Blount AR, Quesenberry KH, Kenworthy KE, Hanna WW. 2011. Tetraploid bahiagrass hybrids: breeding technique, genetic variability and proportion of heterotic hybrids. Euphytica 179: 227–235.
-
- Aliyu OM, Schranz ME, Sharbel TF. 2010. Quantitative variation for apomictic reproduction in the genus Boechera (Brassicaceae). American Journal of Botany 97: 1719–1731. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

