Placental BCRP/ABCG2 Transporter Prevents Fetal Exposure to the Estrogenic Mycotoxin Zearalenone
- PMID: 30576553
- PMCID: PMC6432861
- DOI: 10.1093/toxsci/kfy303
Placental BCRP/ABCG2 Transporter Prevents Fetal Exposure to the Estrogenic Mycotoxin Zearalenone
Abstract
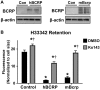
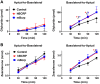
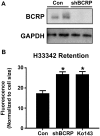
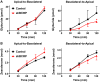
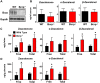
In the placenta, the breast cancer resistance protein (BCRP)/ABCG2 efflux transporter limits the maternal-to-fetal transfer of drugs and chemicals. Previous research has pointed to the estrogenic mycotoxin zearalenone as a potential substrate for BCRP. Here, we sought to assess the role of the BCRP transporter in the transplacental disposition of zearalenone during pregnancy. In vitro transwell transport assays employing BCRP/Bcrp-transfected Madine-Darby canine kidney cells and BeWo trophoblasts with reduced BCRP expression were used to characterize the impact of BCRP on the bidirectional transport of zearalenone. In both models, the presence of BCRP protein increased the basolateral-to-apical transport and reduced the apical-to-basolateral transport of zearalenone over a 2-h period. In vivo pharmacokinetic analyses were then performed using pregnant wild-type and Bcrp-/- mice after a single tail vein injection of zearalenone. Zearalenone and its metabolite α-zearalenol were detectable in serum, placentas, and fetuses from all animals, and β-zearalenol was detected in serum and fetuses, but not placentas. There were no significant differences in the maternal serum concentrations of any analytes between the two genotypes. In Bcrp-/- mice, the free fetal concentrations of zearalenone, α-zearalenol, and β-zearalenol were increased by 115%, 84%, and 150%, respectively, when compared with wild-type mice. Concentrations of free zearalenone and α-zearalenol were elevated 145% and 78% in Bcrp-/- placentas, respectively, when compared with wild-type placentas. Taken together, these data indicate that the placental BCRP transporter functions to reduce the fetal accumulation of zearalenone, which may impact susceptibility to developmental toxicities associated with in utero zearalenone exposure.
Keywords: ABCG2; BCRP; mycotoxin; placenta; zearalenone.
© The Author(s) 2018. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Anandamide down-regulates placental transporter expression through CB2 receptor-mediated inhibition of cAMP synthesis.Pharmacol Res. 2019 Mar;141:331-342. doi: 10.1016/j.phrs.2019.01.002. Epub 2019 Jan 2. Pharmacol Res. 2019. PMID: 30610963 Free PMC article.
-
The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study.Mol Pharmacol. 2008 Mar;73(3):949-59. doi: 10.1124/mol.107.041616. Epub 2007 Dec 13. Mol Pharmacol. 2008. PMID: 18079276
-
Localization of the placental BCRP/ABCG2 transporter to lipid rafts: Role for cholesterol in mediating efflux activity.Placenta. 2017 Jul;55:29-36. doi: 10.1016/j.placenta.2017.04.006. Epub 2017 Apr 12. Placenta. 2017. PMID: 28623970 Free PMC article.
-
An update on expression and function of P-gp/ABCB1 and BCRP/ABCG2 in the placenta and fetus.Expert Opin Drug Metab Toxicol. 2018 Aug;14(8):817-829. doi: 10.1080/17425255.2018.1499726. Epub 2018 Aug 3. Expert Opin Drug Metab Toxicol. 2018. PMID: 30010462 Free PMC article. Review.
-
Placental P-glycoprotein and breast cancer resistance protein: influence of polymorphisms on fetal drug exposure and physiology.Placenta. 2010 May;31(5):351-7. doi: 10.1016/j.placenta.2010.02.010. Epub 2010 Mar 27. Placenta. 2010. PMID: 20347140 Review.
Cited by
-
Regulation of Placental Efflux Transporters during Pregnancy Complications.Drug Metab Dispos. 2022 Oct;50(10):1364-1375. doi: 10.1124/dmd.121.000449. Epub 2022 Jan 6. Drug Metab Dispos. 2022. PMID: 34992073 Free PMC article. Review.
-
Review of the environmental prenatal exposome and its relationship to maternal and fetal health.Reprod Toxicol. 2020 Dec;98:1-12. doi: 10.1016/j.reprotox.2020.02.004. Epub 2020 Feb 23. Reprod Toxicol. 2020. PMID: 32061676 Free PMC article. Review.
-
Breast cancer resistance protein (Bcrp/Abcg2) is selectively modulated by lipopolysaccharide (LPS) in the mouse yolk sac.Reprod Toxicol. 2020 Dec;98:82-91. doi: 10.1016/j.reprotox.2020.09.001. Epub 2020 Sep 8. Reprod Toxicol. 2020. PMID: 32916274 Free PMC article.
-
Optimized extraction and analysis methods using liquid chromatography-tandem mass spectrometry for zearalenone and metabolites in human placental tissue.Heliyon. 2023 Jun 4;9(6):e16940. doi: 10.1016/j.heliyon.2023.e16940. eCollection 2023 Jun. Heliyon. 2023. PMID: 37484340 Free PMC article.
-
In Vitro Evaluation of the Potential Interactions of Zearalenone-14-sulfate and Zearalenone-14-glucuronide with Human Cytochrome P450 Enzymes, Organic Anion Transporting Polypeptides, and ATP-Binding Cassette Multidrug Transporters.ACS Omega. 2025 Jul 16;10(29):32466-32475. doi: 10.1021/acsomega.5c05217. eCollection 2025 Jul 29. ACS Omega. 2025. PMID: 40757271 Free PMC article.
References
-
- An G., Morris M. E. (2011). The sulfated conjugate of biochanin A is a substrate of breast cancer resistant protein (ABCG2). Biopharm. Drug Disp. 32, 446–457. - PubMed
-
- Appelgren L. E., Arora R. G., Larsson P. (1982). Autoradiographic studies of [3h]zearalenone in mice. Toxicology 25, 243–253. - PubMed
-
- Belli P., Bellaton C., Durand J., Balleydier S., Milhau N., Mure M., Mornex J. F., Benahmed M., Le Jan C. (2010). Fetal and neonatal exposure to the mycotoxin zearalenone induces phenotypic alterations in adult rat mammary gland. Food Chem. Toxicol. 48, 2818–2826. - PubMed

