Characterised Flavin-Dependent Two-Component Monooxygenases from the CAM Plasmid of Pseudomonas putida ATCC 17453 (NCIMB 10007): ketolactonases by Another Name
- PMID: 30577535
- PMCID: PMC6352141
- DOI: 10.3390/microorganisms7010001
Characterised Flavin-Dependent Two-Component Monooxygenases from the CAM Plasmid of Pseudomonas putida ATCC 17453 (NCIMB 10007): ketolactonases by Another Name
Abstract
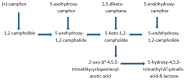
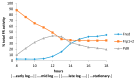
The CAM plasmid-coded isoenzymic diketocamphane monooxygenases induced in Pseudomonas putida ATCC 17453 (NCIMB 10007) by growth of the bacterium on the bicyclic monoterpene (rac)-camphor are notable both for their interesting history, and their strategic importance in chemoenzymatic syntheses. Originally named 'ketolactonase-an enzyme system for cyclic lactonization' because of its characterised mode of action, (+)-camphor-induced 2,5-diketocamphane 1,2-monooxygenase was the first example of a Baeyer-Villiger monooxygenase activity to be confirmed in vitro. Both this enzyme and the enantiocomplementary (-)-camphor-induced 3,6-diketocamphane 1,6-monooxygenase were mistakenly classified and studied as coenzyme-containing flavoproteins for nearly 40 years before being correctly recognised and reinvestigated as FMN-dependent two-component monooxygenases. As has subsequently become evident, both the nature and number of flavin reductases able to supply the requisite reduced flavin co-substrate for the monooxygenases changes progressively throughout the different phases of camphor-dependent growth. Highly purified preparations of the enantiocomplementary monooxygenases have been exploited successfully for undertaking both nucleophilic and electrophilic biooxidations generating various enantiopure lactones and sulfoxides of value as chiral synthons and auxiliaries, respectively. In this review the chequered history, current functional understanding, and scope and value as biocatalysts of the diketocamphane monooxygenases are discussed.
Keywords: diketocamphane monooxygenase; enantiocomplementary enzymes; enantiodivergent biotransformation; flavin-dependent two-component monooxygenase; ketolactonase.
Conflict of interest statement
The author declares no conflict of interest.
Figures












Similar articles
-
The Isoenzymic Diketocamphane Monooxygenases of Pseudomonas putida ATCC 17453-An Episodic History and Still Mysterious after 60 Years.Microorganisms. 2021 Dec 15;9(12):2593. doi: 10.3390/microorganisms9122593. Microorganisms. 2021. PMID: 34946195 Free PMC article. Review.
-
The Role of Dioxygen in Microbial Bio-Oxygenation: Challenging Biochemistry, Illustrated by a Short History of a Long Misunderstood Enzyme.Microorganisms. 2024 Feb 15;12(2):389. doi: 10.3390/microorganisms12020389. Microorganisms. 2024. PMID: 38399793 Free PMC article. Review.
-
Inter-Species Redox Coupling by Flavin Reductases and FMN-Dependent Two-Component Monooxygenases Undertaking Nucleophilic Baeyer-Villiger Biooxygenations.Microorganisms. 2022 Dec 27;11(1):71. doi: 10.3390/microorganisms11010071. Microorganisms. 2022. PMID: 36677363 Free PMC article.
-
Flavin-Dependent Redox Transfers by the Two-Component Diketocamphane Monooxygenases of Camphor-Grown Pseudomonas putida NCIMB 10007.Microorganisms. 2016 Oct 13;4(4):38. doi: 10.3390/microorganisms4040038. Microorganisms. 2016. PMID: 27754389 Free PMC article.
-
Camphor pathway redux: functional recombinant expression of 2,5- and 3,6-diketocamphane monooxygenases of Pseudomonas putida ATCC 17453 with their cognate flavin reductase catalyzing Baeyer-Villiger reactions.Appl Environ Microbiol. 2013 May;79(10):3282-93. doi: 10.1128/AEM.03958-12. Epub 2013 Mar 22. Appl Environ Microbiol. 2013. PMID: 23524667 Free PMC article.
Cited by
-
The Isoenzymic Diketocamphane Monooxygenases of Pseudomonas putida ATCC 17453-An Episodic History and Still Mysterious after 60 Years.Microorganisms. 2021 Dec 15;9(12):2593. doi: 10.3390/microorganisms9122593. Microorganisms. 2021. PMID: 34946195 Free PMC article. Review.
-
The Role of Dioxygen in Microbial Bio-Oxygenation: Challenging Biochemistry, Illustrated by a Short History of a Long Misunderstood Enzyme.Microorganisms. 2024 Feb 15;12(2):389. doi: 10.3390/microorganisms12020389. Microorganisms. 2024. PMID: 38399793 Free PMC article. Review.
-
Inter-Species Redox Coupling by Flavin Reductases and FMN-Dependent Two-Component Monooxygenases Undertaking Nucleophilic Baeyer-Villiger Biooxygenations.Microorganisms. 2022 Dec 27;11(1):71. doi: 10.3390/microorganisms11010071. Microorganisms. 2022. PMID: 36677363 Free PMC article.
-
Conferring the Metabolic Self-Sufficiency of the CAM Plasmid of Pseudomonas putida ATCC 17453: The Key Role of Putidaredoxin Reductase.Microorganisms. 2019 Sep 26;7(10):395. doi: 10.3390/microorganisms7100395. Microorganisms. 2019. PMID: 31561477 Free PMC article.
References
-
- Gunsalus I.C., Marshall V.P. Monoterpene dissimilation. CRC Crit. Rev. Microbiol. 1971;1:291–310. doi: 10.3109/10408417109104484. - DOI
-
- Iwaki H., Grosse S., Bergeron H., Leisch H., Morley K., Hasegawa Y., Lau P.C. Camphor pathway redox: Functional recombinant expression of 2,5- and 3,6-diketocamphane monooxygenases in Pseudomonas putida ATCC 17453 with their cognate flavin reductase catalysing Baeyer-Villiger reactions. Appl. Environ. Microbiol. 2013;79:3282–3293. doi: 10.1128/AEM.03958-12. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

