Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis
- PMID: 30577559
- PMCID: PMC6357192
- DOI: 10.3390/nu11010015
Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis
Abstract
l-Arginine supplementation is a potential therapy for treating cardiovascular and metabolic diseases. However, the use of distinct l-arginine sources, intervened populations, and treatment regimens may have yielded confusion about their efficacy. This research constitutes a systematic review and meta-analysis summarizing the effects of l-arginine supplementation compared to placebo in individuals with cardiovascular disease (CVD), obesity, or diabetes. Eligibility criteria included randomized clinical trials and interventions based on oral supplementation of l-arginine with a minimum duration of three days; comparison groups consisted of individuals with the same disease condition receiving an oral placebo substance. The primary outcome was flow-mediated dilation, and secondary outcomes were nitrite/nitrate (NOx) rate and asymmetric dimethylarginine (ADMA). Statistical heterogeneity among studies included in the meta-analyses was assessed using the inconsistency index (I2). Fifty-four full-text articles from 3761 retrieved references were assessed for eligibility. After exclusions, 13 studies were included for data extraction. There was no difference in blood flow after post-ischemic hyperemia between the supplementation of l-arginine and placebo groups before and after the intervention period (standardized mean difference (SMD) = 0.30; 95% confidence intervals (CIs) = -0.85 to 1.46; I2 = 96%). Sensitivity analysis showed decreased heterogeneity when the studies that most favor arginine and placebo were removed, and positive results in favor of arginine supplementation were found (SMD = 0.59; 95% CIs = 0.10 to 1.08; I2 = 75%). No difference was found in meta-analytical estimates of NOx and ADMA responses between arginine or placebo treatments. Overall, the results indicated that oral l-arginine supplementation was not associated with improvements on selected variables in these patients (PROSPERO Registration: CRD42017077289).
Keywords: asymmetric dimethylarginine; cardiovascular disease; flow-mediated dilation; nitric oxide; obesity; type 2 diabetes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


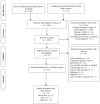


References
-
- Fayh A.P., Krause M., Rodrigues-Krause J., Ribeiro J.L., Ribeiro J.P., Friedman R., Moreira J.C., Reischak-Oliveira A. Effects of L-arginine supplementation on blood flow, oxidative stress status and exercise responses in young adults with uncomplicated type I diabetes. Eur. J. Nutr. 2013;52:975–983. doi: 10.1007/s00394-012-0404-7. - DOI - PubMed
-
- Krause M., Rodrigues-Krause J., O’Hagan C., Medlow P., Davison G., Susta D., Boreham C., Newsholme P., O’Donnell M., Murphy C., et al. The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur. J. Appl. Physiol. 2014;114:251–260. doi: 10.1007/s00421-013-2769-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

