Fungi-Mediated Biotransformation of the Isomeric Forms of the Apocarotenoids Ionone, Damascone and Theaspirane
- PMID: 30577583
- PMCID: PMC6337586
- DOI: 10.3390/molecules24010019
Fungi-Mediated Biotransformation of the Isomeric Forms of the Apocarotenoids Ionone, Damascone and Theaspirane
Abstract
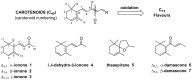
In this work, we describe a study on the biotransformation of seven natural occurring apocarotenoids by means of eleven selected fungal species. The substrates, namely ionone (α-, β- and γ-isomers), 3,4-dehydroionone, damascone (α- and β-isomers) and theaspirane are relevant flavour and fragrances components. We found that most of the investigated biotransformation reactions afforded oxidized products such as hydroxy- keto- or epoxy-derivatives. On the contrary, the reduction of the keto groups or the reduction of the double bond functional groups were observed only for few substrates, where the reduced products are however formed in minor amount. When starting apocarotenoids are isomers of the same chemical compound (e.g., ionone isomers) their biotransformation can give products very different from each other, depending both on the starting substrate and on the fungal species used. Since the majority of the starting apocarotenoids are often available in natural form and the described products are natural compounds, identified in flavours or fragrances, our biotransformation procedures can be regarded as prospective processes for the preparation of high value olfactory active compounds.
Keywords: apocarotenoids; biotransformation; damascone; flavours; fungi; ionone; oxidation; theaspirane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Occurrence of Theaspirane and its Odorant Degradation Products in Hop and Beer.J Agric Food Chem. 2015 Sep 23;63(37):8247-53. doi: 10.1021/acs.jafc.5b03195. Epub 2015 Sep 11. J Agric Food Chem. 2015. PMID: 26321162
-
Selective oxidation of carotenoid-derived aroma compounds by CYP260B1 and CYP267B1 from Sorangium cellulosum So ce56.Appl Microbiol Biotechnol. 2016 May;100(10):4447-57. doi: 10.1007/s00253-015-7269-7. Epub 2016 Jan 15. Appl Microbiol Biotechnol. 2016. PMID: 26767988
-
In Vitro Regio- and Stereoselective Oxidation of β-Ionone by Human Liver Microsomes.Planta Med. 2017 Feb;83(3-04):292-299. doi: 10.1055/s-0042-112128. Epub 2016 Aug 30. Planta Med. 2017. PMID: 27574897
-
Recent Advances in the Synthesis of Carotenoid-Derived Flavours and Fragrances.Molecules. 2015 Jul 15;20(7):12817-40. doi: 10.3390/molecules200712817. Molecules. 2015. PMID: 26184154 Free PMC article. Review.
-
Apocarotenoids: A New Carotenoid-Derived Pathway.Subcell Biochem. 2016;79:239-72. doi: 10.1007/978-3-319-39126-7_9. Subcell Biochem. 2016. PMID: 27485225 Review.
Cited by
-
Bioactive compounds of Curvularia species as a source of various biological activities and biotechnological applications.Front Microbiol. 2022 Dec 8;13:1069095. doi: 10.3389/fmicb.2022.1069095. eCollection 2022. Front Microbiol. 2022. PMID: 36569099 Free PMC article. Review.
-
Enzymes, Biocatalysis and Chemical Biology.Molecules. 2020 May 18;25(10):2354. doi: 10.3390/molecules25102354. Molecules. 2020. PMID: 32443571 Free PMC article.
-
The Relationship between Endophytic Fungi of Chimonanthus praecox and Volatile Metabolites under Different Circadian Rhythms and Blooming Stages.J Fungi (Basel). 2024 Feb 11;10(2):145. doi: 10.3390/jof10020145. J Fungi (Basel). 2024. PMID: 38392817 Free PMC article.
-
Biocatalytic Potential of Native Basidiomycetes from Colombia for Flavour/Aroma Production.Molecules. 2020 Sep 22;25(18):4344. doi: 10.3390/molecules25184344. Molecules. 2020. PMID: 32971920 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

