Combining Radiotherapy and Immunotherapy in Lung Cancer: Can We Expect Limitations Due to Altered Normal Tissue Toxicity?
- PMID: 30577587
- PMCID: PMC6337556
- DOI: 10.3390/ijms20010024
Combining Radiotherapy and Immunotherapy in Lung Cancer: Can We Expect Limitations Due to Altered Normal Tissue Toxicity?
Abstract
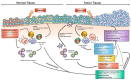
In recent decades, technical advances in surgery and radiotherapy, as well as breakthroughs in the knowledge on cancer biology, have helped to substantially improve the standard of cancer care with respect to overall response rates, progression-free survival, and the quality of life of cancer patients. In this context, immunotherapy is thought to have revolutionized the standard of care for cancer patients in the long term. For example, immunotherapy approaches such as immune checkpoint blockade are currently increasingly being used in cancer treatment, either alone or in combination with chemotherapy or radiotherapy, and there is hope from the first clinical trials that the appropriate integration of immunotherapy into standard care will raise the success rates of cancer therapy to a new level. Nevertheless, successful cancer therapy remains a major challenge, particularly in tumors with either pronounced resistance to chemotherapy and radiation treatment, a high risk of normal tissue complications, or both, as in lung cancer. Chemotherapy, radiotherapy and immunotherapy have the capacity to evoke adverse effects in normal tissues when administered alone. However, therapy concepts are usually highly complex, and it is still not clear if combining immunotherapy with radio(chemo)therapy will increase the risk of normal tissue complications, in particular since normal tissue toxicity induced by chemotherapy and radiotherapy can involve immunologic processes. Unfortunately, no reliable biomarkers are available so far that are suited to predict the unique normal tissue sensitivity of a given patient to a given treatment. Consequently, clinical trials combining radiotherapy and immunotherapy are attracting major attention, not only regarding efficacy, but also with regard to safety. In the present review, we summarize the current knowledge of radiation-induced and immunotherapy-induced effects in tumor and normal tissue of the lung, and discuss the potential limitations of combined radio-immunotherapy in lung cancer with a focus on the suspected risk for enhanced acute and chronic normal tissue toxicity.
Keywords: CTLA-4; Irradiation; PD-L1; PD1; T cells; adverse effects; fibrosis; immune checkpoint inhibition; pneumonitis; pneumopathy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Auperin A., Le Pechoux C., Rolland E., Curran W.J., Furuse K., Fournel P., Belderbos J., Clamon G., Ulutin H.C., Paulus R., et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. - DOI - PubMed
-
- Crabtree T.D., Denlinger C.E., Meyers B.F., El Naqa I., Zoole J., Krupnick A.S., Kreisel D., Patterson G.A., Bradley J.D. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2010;140:377–386. doi: 10.1016/j.jtcvs.2009.12.054. - DOI - PubMed
-
- Onishi H., Shirato H., Nagata Y., Hiraoka M., Fujino M., Gomi K., Karasawa K., Hayakawa K., Niibe Y., Takai Y., et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: Can SBRT be comparable to surgery? Int. J. Radiat. Oncol. Biol. Phys. 2011;81:1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

