PISTILLATA paralogs in Tarenaya hassleriana have diverged in interaction specificity
- PMID: 30577806
- PMCID: PMC6303913
- DOI: 10.1186/s12870-018-1574-0
PISTILLATA paralogs in Tarenaya hassleriana have diverged in interaction specificity
Abstract
Background: Floral organs are specified by MADS-domain transcription factors that act in a combinatorial manner, as summarized in the (A)BCE model. However, this evolutionarily conserved model is in contrast to a remarkable amount of morphological diversity in flowers. One of the mechanisms suggested to contribute to this diversity is duplication of floral MADS-domain transcription factors. Although gene duplication is often followed by loss of one of the copies, sometimes both copies are retained. If both copies are retained they will initially be redundant, providing freedom for one of the paralogs to change function. Here, we examine the evolutionary fate and functional consequences of a transposition event at the base of the Brassicales that resulted in the duplication of the floral regulator PISTILLATA (PI), using Tarenaya hassleriana (Cleomaceae) as a model system.
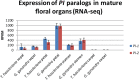
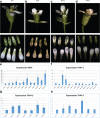
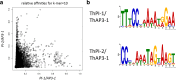
Results: The transposition of a genomic region containing a PI gene led to two paralogs which are located at different positions in the genome. The original PI copy is syntenic in position with most angiosperms, whereas the transposed copy is syntenic with the PI genes in Brassicaceae. The two PI paralogs of T. hassleriana have very similar expression patterns. However, they may have diverged in function, as only one of these PI proteins was able to act heterologously in the first whorl of A. thaliana flowers. We also observed differences in protein complex formation between the two paralogs, and the two paralogs exhibit subtle differences in DNA-binding specificity. Sequence analysis indicates that most of the protein sequence divergence between the two T. hassleriana paralogs emerged in a common ancestor of the Cleomaceae and the Brassicaceae.
Conclusions: We found that the PI paralogs in T. hassleriana have similar expression patterns, but may have diverged at the level of protein function. Data suggest that most protein sequence divergence occurred rapidly, prior to the origin of the Brassicaceae and Cleomaceae. It is tempting to speculate that the interaction specificities of the Brassicaceae-specific PI proteins are different compared to the PI found in other angiosperms. This could lead to PI regulating partly different genes in the Brassicaceae, and ultimately might result in change floral in morphology.
Keywords: Cleomaceae; Flower development; Gene duplications; MADS; PISTILLATA; Paralogs; Tarenaya.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Ohno S. Evolution by gene duplication. 1970.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

