Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis
- PMID: 30577837
- PMCID: PMC6303974
- DOI: 10.1186/s40425-018-0477-9
Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis
Abstract
Background: Immune-checkpoint inhibitors plus chemotherapy are emerging as effective first-line treatment in advanced non-small-cell lung carcinoma (NSCLC), but little is known about the magnitude of benefits and potential clinical predictors.
Methods: We performed a meta-analysis of randomized trials that compared PD-1/PD-L1 inhibitor plus chemotherapy with chemotherapy in first line of treatment for advanced NSCLC. The outcomes included progression-free survival (PFS), overall survival (OS), objective response rate (ORR) and treatment-related adverse events (AEs). A fixed-effect or random-effects model was adopted depending on between-study heterogeneity.
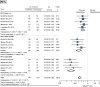
Results: Six trials involving 3144 patients were included. PD-1/PD-L1 inhibitor plus chemotherapy was significantly associated with improvement of PFS (hazards ratio [HR], 0.62; 95% CI 0.57-0.67; P < .001), OS (HR, 0.68; 95% CI 0.53-0.87; P = .002) and ORR (relative ratio [RR], 1.56; 95% CI 1.29-1.89; P < .001), irrespective of PD-L1 expression level. The significant predictor(s) for treatment benefit with combination therapy versus chemotherapy alone were PD-L1 expression level for PFS (P < .001); types of checkpoint inhibitor for ORR (P < .001); histology (P = .025), age (P = .038), gender (P < .001), and types of checkpoint inhibitor (P < .001) for OS. In safety analyses, PD-1/PD-L1 inhibitor plus chemotherapy had significantly higher incidence of adverse events (AEs) of grade 3 or higher (RR, 1.14; P = .007), AEs leading to treatment discontinuation (RR, 1.29; P = .022), serious AEs (RR 1.70; P = .006), immune mediated AEs of any grade (RR, 2.37; P < .001), and immune mediated AEs of grade 3 or higher (RR, 3.71; P < .001).
Conclusions: PD-1/PD-L1 inhibitor plus chemotherapy, compared with chemotherapy, is associated with significantly improved PFS, ORR, and OS in first-line therapy in NSCLC, at the expense of increased treatment-related AEs.
Keywords: Chemotherapy; Immune checkpoint inhibitor; Non-small cell lung carcinoma; Predict; Programmed death 1; Programmed death 1 ligand 1.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Li Zhang has received research support from AstraZeneca, Eli Lilly and company, and Roche. No other disclosures were reported.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Global Burden of Disease Cancer C, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553–1568. - PMC - PubMed
-
- Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
