Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism
- PMID: 30578168
- PMCID: PMC6358549
- DOI: 10.1016/j.molmet.2018.12.001
Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism
Abstract
Objective: Structurally-improved GIP analogs were developed to determine precisely whether GIP receptor (GIPR) agonism or antagonism lowers body weight in obese mice.
Methods: A series of peptide-based GIP analogs, including structurally diverse agonists and a long-acting antagonist, were generated and characterized in vitro using functional assays in cell systems overexpressing human and mouse derived receptors. These analogs were characterized in vivo in DIO mice following acute dosing for effects on glycemic control, and following chronic dosing for effects on body weight and food intake. Pair-feeding studies and indirect calorimetry were used to survey the mechanism for body weight lowering. Congenital Gipr-/- and Glp1r-/- DIO mice were used to investigate the selectivity of the agonists and to ascribe the pharmacology to effects mediated by the GIPR.
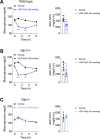
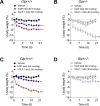
Results: Non-acylated, Aib2 substituted analogs derived from human GIP sequence showed full in vitro potency at human GIPR and subtly reduced in vitro potency at mouse GIPR without cross-reactivity at GLP-1R. These GIPR agonists lowered acute blood glucose in wild-type and Glp1r-/- mice, and this effect was absent in Gipr-/- mice, which confirmed selectivity towards GIPR. Chronic treatment of DIO mice resulted in modest yet consistent, dose-dependent decreased body weight across many studies with diverse analogs. The mechanism for body weight lowering is due to reductions in food intake, not energy expenditure, as suggested by pair-feeding studies and indirect calorimetry assessment. The weight lowering effect was preserved in DIO Glp-1r-/- mice and absent in DIO Gipr-/- mice. The body weight lowering efficacy of GIPR agonists was enhanced with analogs that exhibit higher mouse GIPR potency, with increased frequency of administration, and with fatty-acylated peptides of extended duration of action. Additionally, a fatty-acylated, N-terminally truncated GIP analog was shown to have high in vitro antagonism potency for human and mouse GIPR without cross-reactive activity at mouse GLP-1R or mouse glucagon receptor (GcgR). This acylated antagonist sufficiently inhibited the acute effects of GIP to improve glucose tolerance in DIO mice. Chronic treatment of DIO mice with high doses of this acylated GIPR antagonist did not result in body weight change. Further, co-treatment of this acylated GIPR antagonist with liraglutide, an acylated GLP-1R agonist, to DIO mice did not result in increased body weight lowering relative to liraglutide-treated mice. Enhanced body weight lowering in DIO mice was evident however following co-treatment of long-acting selective individual agonists for GLP-1R and GIPR, consistent with previous data.
Conclusions: We conclude that peptide-based GIPR agonists, not peptide-based GIPR antagonists, that are suitably optimized for receptor selectivity, cross-species activity, and duration of action consistently lower body weight in DIO mice, although with moderate efficacy relative to GLP-1R agonists. These preclinical rodent pharmacology results, in accordance with recent clinical results, provide definitive proof that systemic GIPR agonism, not antagonism, is beneficial for body weight loss.
Keywords: Agonism; Diet-induced obese (DIO) mice; Glucose-dependent insulinotropic polypeptide (GIP); Obesity; Pharmacology.
Copyright © 2018 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures



References
-
- Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2016;375(19):1834–1844. - PubMed
-
- Nauck M.A., Heimesaat M.M., Orskov C., Holst J.J., Ebert R., Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. Journal of Clinical Investigation. 1993;91(1):301–307. - PMC - PubMed
-
- Hojberg P.V., Vilsboll T., Rabol R., Knop F.K., Bache M., Krarup T. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52(2):199–207. - PubMed
-
- Finan B., Ma T., Ottaway N., Muller T.D., Habegger K.M., Heppner K.M. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Science Translational Medicine. 2013;5(209):209ra151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

