A Web-Based Telemanagement System for Patients With Complex Inflammatory Bowel Disease: Protocol for a Randomized Controlled Clinical Trial
- PMID: 30578197
- PMCID: PMC6320427
- DOI: 10.2196/resprot.9639
A Web-Based Telemanagement System for Patients With Complex Inflammatory Bowel Disease: Protocol for a Randomized Controlled Clinical Trial
Abstract
Background: Telemedicine has been successfully used to provide inflammatory bowel disease (IBD) patients with health care services remotely via the implementation of information and communications technology, which uses safe and feasible apps that have been well accepted by patients in remission. However, the design of telemedicine apps in this setting involves difficulties that hinder the adherence of patients to the follow-up plans and the efficacy of these systems to improve disease activity and quality of life.
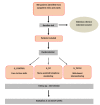
Objective: This study aimed to evaluate the development of a Web platform, Telemonitoring of Crohn Disease and Ulcerative Colitis (TECCU), for remote monitoring of patients with complex IBD and the design of a clinical trial involving IBD patients who received standard care (G_Control), nurse-assisted telephone care (G_NT), or care based on distance monitoring (G_TECCU).
Methods: We describe the development of a remote monitoring system and the difficulties encountered in designing the platform. A 3-arm randomized controlled trial was designed to evaluate the effectiveness of this Web platform in disease management compared with G_NT and G_Control.
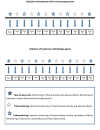
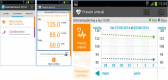
Results: According to the schedules established for the medical treatment initiated (corticosteroids, immunosuppressants, or biological agents), a total of 63 patients (21 patients from each group) answered periodic questionnaires regarding disease activity, quality of life, therapeutic adherence, adverse effects, satisfaction, work productivity, and social activities. Blood and stool analyses (fecal calprotectin) were performed periodically. On the basis of the results of these tests in G_TECCU, alerts were generated in a Web platform with adapted action plans, including changes in medication and frequency of follow-up. The main issues found were the development of an easy-to-use Web platform, the selection of validated clinical scores and objective biomarkers for remote monitoring, and the design of a clinical trial to compare the 3 main follow-up methods evaluated to date in IBD.
Conclusions: The development of a Web-based remote management program for safe and adequate control of IBD proved challenging. The results of this clinical trial will advance knowledge regarding the effectiveness of TECCU Web platform for improvement of disease activity, quality of life, and use of health care resources in complex IBD patients.
Trial registration: ClinicalTrials.gov NCT02943538; https://clinicaltrials.gov/ct2/show/NCT02943538 (Archived by WebCite at http://www.webcitation.org/6y4DQdmt8).
International registered report identifier (irrid): RR1-10.2196/9639.
Keywords: Crohn disease; eHealth; inflammatory bowel disease; information technology; telemedicine; ulcerative colitis.
©Mariam Aguas, Javier del Hoyo, Raquel Faubel, Diana Muñoz, David Domínguez, Guillermo Bastida, Belén Navarro, Alejandra Barrios, Bernardo Valdivieso, Marisa Correcher, Pilar Nos. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 21.12.2018.
Conflict of interest statement
Conflicts of Interest: DD is the General Manager of Connected Health Services, the company that owns the intellectual property and commercial rights of the NOMHADchronic software platform, which was configured and used in the TECCU trial for the remote management of patients with CD and UC.
Figures

References
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. - DOI - PubMed
-
- López-Serrano P, Pérez-Calle JL, Carrera-Alonso E, Pérez-Fernández T, Rodríguez-Caravaca G, Boixeda-de-Miguel D, Fernández-Rodríguez CM. Epidemiologic study on the current incidence of inflammatory bowel disease in Madrid. Rev Esp Enferm Dig. 2009 Nov;101(11):768–72. http://www.grupoaran.com/mrmUpdate/lecturaPDFfromXML.asp?IdArt=4618354&T... - PubMed
-
- Casellas F, Arenas JI, Baudet JS, Fábregas S, García N, Gelabert J, Medina C, Ochotorena I, Papo M, Rodrigo L, Malagelada J. Impairment of health-related quality of life in patients with inflammatory bowel disease: a Spanish multicenter study. Inflamm Bowel Dis. 2005 May;11(5):488–96. doi: 10.1097/01.MIB.0000159661.55028.56. - DOI - PubMed
-
- Huppertz-Hauss G, Høivik ML, Langholz E, Odes S, Småstuen M, Stockbrugger R, Hoff G, Moum B, Bernklev T. Health-related quality of life in inflammatory bowel disease in a European-wide population-based cohort 10 years after diagnosis. Inflamm Bowel Dis. 2015 Feb;21(2):337–44. doi: 10.1097/MIB.0000000000000272. http://europepmc.org/abstract/MED/25569735 - DOI - PMC - PubMed
-
- Kappelman MD, Porter CQ, Galanko JA, Rifas-Shiman SL, Ollendorf DA, Sandler RS, Finkelstein JA. Utilization of healthcare resources by US children and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2011 Jan;17(1):62–8. doi: 10.1002/ibd.21371. http://europepmc.org/abstract/MED/20564532 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

