Competition among Nasal Bacteria Suggests a Role for Siderophore-Mediated Interactions in Shaping the Human Nasal Microbiota
- PMID: 30578265
- PMCID: PMC6498180
- DOI: 10.1128/AEM.02406-18
Competition among Nasal Bacteria Suggests a Role for Siderophore-Mediated Interactions in Shaping the Human Nasal Microbiota
Abstract
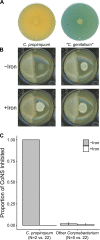
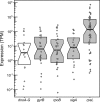
Resources available in the human nasal cavity are limited. Therefore, to successfully colonize the nasal cavity, bacteria must compete for scarce nutrients. Competition may occur directly through interference (e.g., antibiotics) or indirectly by nutrient sequestration. To investigate the nature of nasal bacterial competition, we performed coculture inhibition assays between nasal Actinobacteria and Staphylococcus spp. We found that isolates of coagulase-negative staphylococci (CoNS) were sensitive to growth inhibition by Actinobacteria but that Staphylococcus aureus isolates were resistant to inhibition. Among Actinobacteria, we observed that Corynebacterium spp. were variable in their ability to inhibit CoNS. We sequenced the genomes of 10 Corynebacterium species isolates, including 3 Corynebacterium propinquum isolates that strongly inhibited CoNS and 7 other Corynebacterium species isolates that only weakly inhibited CoNS. Using a comparative genomics approach, we found that the C. propinquum genomes were enriched in genes for iron acquisition and harbored a biosynthetic gene cluster (BGC) for siderophore production, absent in the noninhibitory Corynebacterium species genomes. Using a chrome azurol S assay, we confirmed that C. propinquum produced siderophores. We demonstrated that iron supplementation rescued CoNS from inhibition by C. propinquum, suggesting that inhibition was due to iron restriction through siderophore production. Through comparative metabolomics and molecular networking, we identified the siderophore produced by C. propinquum as dehydroxynocardamine. Finally, we confirmed that the dehydroxynocardamine BGC is expressed in vivo by analyzing human nasal metatranscriptomes from the NIH Human Microbiome Project. Together, our results suggest that bacteria produce siderophores to compete for limited available iron in the nasal cavity and improve their fitness.IMPORTANCE Within the nasal cavity, interference competition through antimicrobial production is prevalent. For instance, nasal Staphylococcus species strains can inhibit the growth of other bacteria through the production of nonribosomal peptides and ribosomally synthesized and posttranslationally modified peptides. In contrast, bacteria engaging in exploitation competition modify the external environment to prevent competitors from growing, usually by hindering access to or depleting essential nutrients. As the nasal cavity is a nutrient-limited environment, we hypothesized that exploitation competition occurs in this system. We determined that Corynebacterium propinquum produces an iron-chelating siderophore, and this iron-sequestering molecule correlates with the ability to inhibit the growth of coagulase-negative staphylococci. Furthermore, we found that the genes required for siderophore production are expressed in vivo Thus, although siderophore production by bacteria is often considered a virulence trait, our work indicates that bacteria may produce siderophores to compete for limited iron in the human nasal cavity.
Keywords: Actinobacteria; Corynebacterium; Staphylococcus; competition; dehydroxynocardamine; iron; nasal microbiome; siderophore.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Nasal commensals reduce Staphylococcus aureus proliferation by restricting siderophore availability.ISME J. 2024 Jan 8;18(1):wrae123. doi: 10.1093/ismejo/wrae123. ISME J. 2024. PMID: 38987933 Free PMC article.
-
Corynebacterium pseudodiphtheriticum Exploits Staphylococcus aureus Virulence Components in a Novel Polymicrobial Defense Strategy.mBio. 2019 Jan 8;10(1):e02491-18. doi: 10.1128/mBio.02491-18. mBio. 2019. PMID: 30622190 Free PMC article.
-
Production of siderophore by coagulase-negative staphylococci and its relation to virulence.Eur J Clin Microbiol Infect Dis. 1994 Dec;13(12):1063-6. doi: 10.1007/BF02111829. Eur J Clin Microbiol Infect Dis. 1994. PMID: 7889970
-
Siderophore-mediated iron acquisition and modulation of host-bacterial interactions.Free Radic Biol Med. 2017 Apr;105:68-78. doi: 10.1016/j.freeradbiomed.2016.10.489. Epub 2016 Oct 22. Free Radic Biol Med. 2017. PMID: 27780750 Free PMC article. Review.
-
Siderophores in Iron Metabolism: From Mechanism to Therapy Potential.Trends Mol Med. 2016 Dec;22(12):1077-1090. doi: 10.1016/j.molmed.2016.10.005. Epub 2016 Nov 4. Trends Mol Med. 2016. PMID: 27825668 Free PMC article. Review.
Cited by
-
The ubiquitous catechol moiety elicits siderophore and angucycline production in Streptomyces.Commun Chem. 2022 Feb 3;5(1):14. doi: 10.1038/s42004-022-00632-4. Commun Chem. 2022. PMID: 36697563 Free PMC article.
-
Staphylococcus aureus: A Review of the Pathogenesis and Virulence Mechanisms.Antibiotics (Basel). 2025 May 6;14(5):470. doi: 10.3390/antibiotics14050470. Antibiotics (Basel). 2025. PMID: 40426537 Free PMC article. Review.
-
Respiratory and Neurological Disease across Different Ethnic Groups Is Influenced by the Microbiome.Microorganisms. 2021 Sep 16;9(9):1965. doi: 10.3390/microorganisms9091965. Microorganisms. 2021. PMID: 34576860 Free PMC article. Review.
-
The Upper Airway Microbiota, Environmental Exposures, Inflammation, and Disease.Medicina (Kaunas). 2021 Aug 14;57(8):823. doi: 10.3390/medicina57080823. Medicina (Kaunas). 2021. PMID: 34441029 Free PMC article. Review.
-
Antimicrobial Resistance in Escherichia coli Strains Isolated from Humans and Pet Animals.Antibiotics (Basel). 2021 Jan 13;10(1):69. doi: 10.3390/antibiotics10010069. Antibiotics (Basel). 2021. PMID: 33450827 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

