Epilepsy-associated mutations in the voltage sensor of KCNQ3 affect voltage dependence of channel opening
- PMID: 30578330
- PMCID: PMC6363412
- DOI: 10.1085/jgp.201812221
Epilepsy-associated mutations in the voltage sensor of KCNQ3 affect voltage dependence of channel opening
Abstract
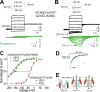
One of the major factors known to cause neuronal hyperexcitability is malfunction of the potassium channels formed by KCNQ2 and KCNQ3. These channel subunits underlie the M current, which regulates neuronal excitability. Here, I investigate the molecular mechanisms by which epilepsy-associated mutations in the voltage sensor (S4) of KCNQ3 cause channel malfunction. Voltage clamp fluorometry reveals that the R230C mutation in KCNQ3 allows S4 movement but shifts the open/closed transition of the gate to very negative potentials. This results in the mutated channel remaining open throughout the physiological voltage range. Substitution of R230 with natural and unnatural amino acids indicates that the functional effect of the arginine residue at position 230 depends on both its positive charge and the size of its side chain. I find that KCNQ3-R230C is hard to close, but it is capable of being closed at strong negative voltages. I suggest that compounds that shift the voltage dependence of S4 activation to more positive potentials would promote gate closure and thus have therapeutic potential.
© 2019 Barro-Soria.
Figures


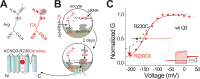

Comment in
-
Investigating an epileptogenic mutation.J Gen Physiol. 2019 Feb 4;151(2):96. doi: 10.1085/jgp.201812316. Epub 2019 Jan 9. J Gen Physiol. 2019. PMID: 30626616 Free PMC article.
References
-
- Bartos D.C., Duchatelet S., Burgess D.E., Klug D., Denjoy I., Peat R., Lupoglazoff J.M., Fressart V., Berthet M., Ackerman M.J., et al. . 2011. R231C mutation in KCNQ1 causes long QT syndrome type 1 and familial atrial fibrillation. Heart Rhythm. 8:48–55. 10.1016/j.hrthm.2010.09.010 - DOI - PMC - PubMed

