Acute exacerbations of progressive-fibrosing interstitial lung diseases
- PMID: 30578331
- PMCID: PMC9488799
- DOI: 10.1183/16000617.0071-2018
Acute exacerbations of progressive-fibrosing interstitial lung diseases
Abstract
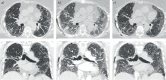
Acute exacerbation of interstitial lung disease (ILD) is associated with a poor prognosis and high mortality. Numerous studies have documented acute exacerbation in idiopathic pulmonary fibrosis (IPF), but less is known about these events in other ILDs that may present a progressive-fibrosing phenotype. We propose defining acute exacerbation as an acute, clinically significant respiratory deterioration, typically less than 1 month in duration, together with computerised tomography imaging showing new bilateral glass opacity and/or consolidation superimposed on a background pattern consistent with fibrosing ILDs. Drawing on observations in IPF, it is suspected that epithelial injury or proliferation and autoimmunity are risk factors for acute exacerbation in ILDs that may present a progressive-fibrosing phenotype, but further studies are required. Current acute exacerbation management strategies are based on recommendations in IPF, but no randomised controlled trials of acute exacerbation management have been performed. Although there are no formal strategies to prevent the development of acute exacerbation, possible approaches include antifibrotic drugs (such as nintedanib and pirfenidone), and minimising exposure to infection, airborne irritants and pollutants. This review discusses the current knowledge of acute exacerbation of ILDs that may present a progressive-fibrosing phenotype and acknowledges limitations of the data available.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: M. Kolb reports grants and personal fees from Roche, Boehringer Ingelheim and Prometic, personal fees from Gilead and Genoa, and grants from Actelion, Respivert, Alkermes and Pharmaxis, outside the submitted work. Conflict of interest: B. Bondue reports grants and personal fees from Boehringer Ingelheim and Le Roche-Hoffmann-La Roche, outside the submitted work. Conflict of interest: A. Pesci has nothing to disclose. Conflict of interest: Y. Miyazaki reports grants and personal fees from Nippon Boehringer Ingelheim and grants from Shionogi, outside the submitted work. Conflict of interest: J.W. Song has nothing to disclose. Conflict of interest: N.Y. Bhatt has nothing to disclose. Conflict of interest: J.T. Huggins reports grants and other fees from Roche/Genentech and Boehringer Ingelheim, during the conduct of the study. Conflict of interest: J.M. Oldham reports other support from Boehringer Ingelheim for writing fees, during the conduct of the study; and personal fees (for speakers fees and advisory board fees) from Boehringer Ingelheim and Genentech, outside the submitted work. Conflict of interest: M.L. Padilla reports personal fees from Boehringer Ingelheim and Genentech, outside the submitted work. Conflict of interest: J. Roman reports grants and personal fees from Boehringer Ingelheim and is a member of the Medical Advisory Board of Pulmonary Fibrosis Foundation. Conflict of interest: S. Shapera reports personal fees from AstraZeneca, grants, personal fees (participation in clinical trials) and other from Boehringer Ingelheim, Canada and Hoffman La-Roche, Canada, and other (participation in clinical trials) from Medimmune, ProMetic Canada and Sanofi-Aventis, outside the submitted work.
Figures
Comment in
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
