Tuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant
- PMID: 30578352
- PMCID: PMC6341984
- DOI: 10.1126/sciimmunol.aau8714
Tuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant
Abstract
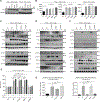
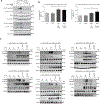
Inherited IL-12Rβ1 and TYK2 deficiencies impair both IL-12- and IL-23-dependent IFN-γ immunity and are rare monogenic causes of tuberculosis, each found in less than 1/600,000 individuals. We show that homozygosity for the common TYK2 P1104A allele, which is found in about 1/600 Europeans and between 1/1000 and 1/10,000 individuals in regions other than East Asia, is more frequent in a cohort of patients with tuberculosis from endemic areas than in ethnicity-adjusted controls (P = 8.37 × 10-8; odds ratio, 89.31; 95% CI, 14.7 to 1725). Moreover, the frequency of P1104A in Europeans has decreased, from about 9% to 4.2%, over the past 4000 years, consistent with purging of this variant by endemic tuberculosis. Surprisingly, we also show that TYK2 P1104A impairs cellular responses to IL-23, but not to IFN-α, IL-10, or even IL-12, which, like IL-23, induces IFN-γ via activation of TYK2 and JAK2. Moreover, TYK2 P1104A is properly docked on cytokine receptors and can be phosphorylated by the proximal JAK, but lacks catalytic activity. Last, we show that the catalytic activity of TYK2 is essential for IL-23, but not IL-12, responses in cells expressing wild-type JAK2. In contrast, the catalytic activity of JAK2 is redundant for both IL-12 and IL-23 responses, because the catalytically inactive P1057A JAK2, which is also docked and phosphorylated, rescues signaling in cells expressing wild-type TYK2. In conclusion, homozygosity for the catalytically inactive P1104A missense variant of TYK2 selectively disrupts the induction of IFN-γ by IL-23 and is a common monogenic etiology of tuberculosis.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Declaration of interest
The authors declare no competing interests.
Figures
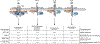



References
-
- Borgdorff MW et al., The incubation period distribution of tuberculosis estimated with a molecular epidemiological approach. Int J Epidemiol 40, 964–970 (2011). - PubMed
-
- WHO, Global tuberculosis report WHO/HTM/TB/2015.22, (2015).
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR021905/RR/NCRR NIH HHS/United States
- T32 GM066699/GM/NIGMS NIH HHS/United States
- U01 AI088685/AI/NIAID NIH HHS/United States
- UL1 TR001866/TR/NCATS NIH HHS/United States
- S10 OD018521/OD/NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 AI095983/AI/NIAID NIH HHS/United States
- K99 AI127932/AI/NIAID NIH HHS/United States
- R01 AI089970/AI/NIAID NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
- U24 HG008956/HG/NHGRI NIH HHS/United States
- P20 GM103496/GM/NIGMS NIH HHS/United States
- U19 AI111143/AI/NIAID NIH HHS/United States
- UM1 HG006504/HG/NHGRI NIH HHS/United States
- R01 AI130345/AI/NIAID NIH HHS/United States
- P30 GM118228/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

