Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis
- PMID: 30578394
- PMCID: PMC6422836
- DOI: 10.1183/13993003.00663-2018
Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis
Abstract
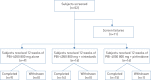
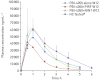
PBI-4050 is a novel orally active small-molecule compound with demonstrated anti-fibrotic activity in several models of fibrosis, including lung fibrosis. We present results from our first clinical study of PBI-4050 in patients with idiopathic pulmonary fibrosis (IPF).This 12-week open-label study explored the safety, efficacy and pharmacokinetics of daily oral doses of 800 mg PBI-4050 alone and in combination with nintedanib or pirfenidone in patients with predominantly mild or moderate IPF. Nine patients received PBI-4050 alone, 16 patients received PBI-4050 with nintedanib and 16 patients received PBI-4050 with pirfenidone.PBI-4050 alone or in combination with nintedanib or pirfenidone was well tolerated. Pharmacokinetic profiles for PBI-4050 were similar in the PBI-4050 alone and PBI-4050+nintedanib groups but reduced in the PBI-4050+pirfenidone group, suggesting a drug-drug interaction. There were no significant changes in forced vital capacity (FVC), either in % predicted or mL, from baseline to week 12 for PBI-4050 alone or PBI-4050+nintedanib. In contrast, a statistically significant reduction (p<0.024) in FVC % pred was seen for PBI-4050+pirfenidone after 12 weeks.There were no safety concerns with PBI-4050 alone or in combination with nintedanib or pirfenidone in IPF patients. The stability of FVC between baseline and week 12 looked encouraging for PBI-4050 alone and in combination with nintedanib.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: N. Khalil has nothing to disclose. Conflict of interest: H. Manganas reports receiving grants from Prometic, during the conduct of the study. Conflict of interest: C.J. Ryerson reports clinical trial participation for Prometic, during the conduct of the study; and receiving grants and personal fees from Boehringer Ingelheim and Hoffmann-La Roche, outside the submitted work. Conflict of interest: S. Shapera reports receiving: personal fees for speaking engagements from AstraZeneca; grants and personal fees for participation in clinical trials as site PI, membership of advisory boards, grants for knowledge translation research, and honoraria for speaking engagements from Boehringer Ingelheim Canada; grants and personal fees for participation in clinical trials as site PI, membership of advisory boards, grants for clinical epidemiology research, and honoraria for speaking engagements from Hoffman-La Roche Canada, outside the submitted work. S. Shapera participated in clinical trials as site PI for Medimmune, Prometic Canada and Sanofi-Aventis, outside the submitted work. Conflict of interest: A.M. Cantin reports receiving grants and fees for consulting from Prometic, during the conduct of the study. Conflict of interest: P. Hernandez reports receiving: grants paid to his institution for conduct of research from Prometic, during the conduct of the study; personal fees for advisory board work from Actelion, personal fees for advisory board work and CME talks from AstraZeneca, grants paid to his institution for conduct of research and personal fees for advisory board work from Boehringer Ingelheim, grants paid to his institution for conduct of research from CSL Behring, Grifols and Cyclomedica, personal fees for advisory board work from GSK Novartis, Teva and Trudell, and grants and personal fees for advisory board work and CME talks from Bayer, outside the submitted work. Conflict of interest: E.E. Turcotte has nothing to disclose. Conflict of interest: J.M. Parker was employed by Prometic Life Sciences Inc., during the conduct of the study. Conflict of interest: J.E. Moran reports receiving personal fees, cash and equity considerations as an employee and shareholder of Prometic Life Sciences Inc., outside the submitted work. Conflict of interest: G.R. Albert is an employee of Prometic Life Sciences Inc. Conflict of interest: R. Sawtell was employed by Prometic Life Sciences Inc., during the conduct of the study. Conflict of interest: A. Hagerimana was employed by Prometic Life Sciences Inc., during the conduct of the study. Conflict of interest: P. Laurin reports receiving personal fees, cash and equity considerations as an employee and shareholder of Prometic Life Sciences Inc., outside the submitted work; and in addition, has a patent EP 2427417 issued, a patent EP 2427416 issued and a patent EP 2970088 pending. Conflict of interest: L. Gagnon reports receiving personal fees, cash and equity considerations as employee and shareholder of Prometic Life Sciences Inc., outside the submitted work; and has a patent EP 2427417 issued, a patent EP 2427416 issued and a patent EP 2970088 pending. Conflict of interest: F. Cesari reports receiving personal fees, cash and equity considerations as an employee and shareholder of Prometic Life Sciences Inc., outside the submitted work. Conflict of interest: M. Kolb reports receiving: grants from Prometic, during the conduct of the study; grants and personal fees from Roche, Boehringer Ingelheim and GSK, personal fees from Gilead and Genoa, and grants from Actelion, Respivert, Alkermes and Pharmaxis, outside the submitted work.
Figures

Comment in
-
Molecular endpoints for establishing target engagement by novel idiopathic pulmonary fibrosis therapies.Eur Respir J. 2019 Mar 18;53(3):1900283. doi: 10.1183/13993003.00283-2019. Print 2019 Mar. Eur Respir J. 2019. PMID: 30886027 No abstract available.
References
-
- King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 2011; 378: 1949–1961. - PubMed
-
- Hutchinson J, Fogarty A, Hubbard R, et al. . Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J 2015; 46: 795–806. - PubMed
-
- Hopkins RB, Burke N, Fell C, et al. . Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J 2016; 48: 187–195. - PubMed
-
- Marshall DC, Salciccioli JD, Shea BS, et al. . Trends in mortality from idiopathic pulmonary fibrosis in the European Union: an observational study of the WHO mortality database from 2001–2013. Eur Respir J 2018; 51: 1701603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
