Comparative effectiveness of exenatide once-weekly versus liraglutide in routine clinical practice: A retrospective multicentre study and meta-analysis of observational studies
- PMID: 30578607
- PMCID: PMC6590315
- DOI: 10.1111/dom.13623
Comparative effectiveness of exenatide once-weekly versus liraglutide in routine clinical practice: A retrospective multicentre study and meta-analysis of observational studies
Abstract
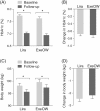
In this study, we retrospectively compared the effectiveness of exenatide once-weekly (ExeOW) versus liraglutide in non-insulin treated patients with type 2 diabetes followed under routine care. We also present a meta-analysis of similar observational studies available in the literature. In our multicentre retrospective study, patients initiating ExeOW (n = 204) or liraglutide (n = 410) had similar baseline clinical characteristics. Change in HbA1c at 6 months was superimposable in the two groups (-0.7% ± 1.0%), and changes in body weight were also similar (ExeOW -2.2 ± 3.7 kg; liraglutide -2.5 ± 4.3 kg; p = 0.457). Discontinuation rates were numerically but not significantly lower for ExeOW versus liraglutide. Pooling these data with those of observational studies available in the literature yielded superimposable effects between the two groups for the change in HbA1c and body weight, with a higher risk of discontinuation (mainly based on pharmacy refill rates) for ExeOW. We conclude that, in patients under routine care, initiation of ExeOW provides similar benefits on HbA1c and body weight as initiation of liraglutide. These data help view the results of randomized controlled trials from the perspective of their application in routine clinical practice.
Keywords: antidiabetic drug; cohort study; exenatide; liraglutide; type 2 diabetes.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Author contributions
Study design: G.P.F. and A.A. Data collection and analysis: G.P.F., B.M.B., A.L., B.F., P.S.M. and N.S. Manuscript writing: G.P.F and A.A. Manuscript revision: G.P.F., B.M.B., A.L., B.F., P.S.M., N.S. and A.A. All authors approved the final version of the manuscript.
Figures

References
-
- Aroda VR. A review of GLP‐1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20(suppl 1):22‐33. - PubMed
-
- Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519‐1529. - PubMed
-
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

