Astrocytic changes with aging and Alzheimer's disease-type pathology in chimpanzees
- PMID: 30578640
- PMCID: PMC6401278
- DOI: 10.1002/cne.24610
Astrocytic changes with aging and Alzheimer's disease-type pathology in chimpanzees
Erratum in
-
Erratum.J Comp Neurol. 2021 May 1;529(7):1719. doi: 10.1002/cne.24755. J Comp Neurol. 2021. PMID: 33748970 No abstract available.
Abstract
Astrocytes are the main homeostatic cell of the central nervous system. In addition, astrocytes mediate an inflammatory response when reactive to injury or disease known as astrogliosis. Astrogliosis is marked by an increased expression of glial fibrillary acidic protein (GFAP) and cellular hypertrophy. Some degree of astrogliosis is associated with normal aging and degenerative conditions such as Alzheimer's disease (AD) and other dementing illnesses in humans. The recent observation of pathological markers of AD (amyloid plaques and neurofibrillary tangles) in aged chimpanzee brains provided an opportunity to examine the relationships among aging, AD-type pathology, and astrocyte activation in our closest living relatives. Stereologic methods were used to quantify GFAP-immunoreactive astrocyte density and soma volume in layers I, III, and V of the prefrontal and middle temporal cortex, as well as in hippocampal fields CA1 and CA3. We found that the patterns of astrocyte activation in the aged chimpanzee brain are distinct from humans. GFAP expression does not increase with age in chimpanzees, possibly indicative of lower oxidative stress loads. Similar to humans, chimpanzee layer I astrocytes in the prefrontal cortex are susceptible to AD-like changes. Both prefrontal cortex layer I and hippocampal astrocytes exhibit a high degree of astrogliosis that is positively correlated with accumulation of amyloid beta and tau proteins. However, unlike humans, chimpanzees do not display astrogliosis in other cortical layers. These results demonstrate a unique pattern of cortical aging in chimpanzees and suggest that inflammatory processes may differ between humans and chimpanzees in response to pathology.
Keywords: Alzheimer's disease; RRID: AB_2109645; RRID: AB_223647; RRID: AB_2313952; RRID: AB_2314223; aging; astrocytes; cerebral cortex; chimpanzees; hippocampus; prefrontal cortex; stereology.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures

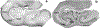


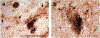

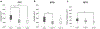
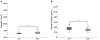




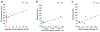
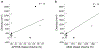
References
-
- Almkvist O, Basun H, Bäckman L, Herlitz A, Lannfelt L, Small B, …Winblad B (1998). Mild cognitive impairment: an early stage of Alzheimer’s disease? Journal of Neural Transmission 54, 21–29. - PubMed
-
- Alzheimer A (1911). Über eigenartige Krankheitsfälle des späteren Alters. Zeitschrift der Gesellschaft für Neurologie und Psychiatrie 4, 356–385.
-
- Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M, & Kalaria RN (2013). Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer’s disease and vascular dementia. Current Alzheimer’s Research 10, 642–653. - PubMed
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, & Van Hosen GW (1991). The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex 1, 103–116. - PubMed
-
- Armstrong RA (2009). The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer’s disease. Folia Neuropathologica 47, 289–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

