IFN-γ and CD25 drive distinct pathologic features during hemophagocytic lymphohistiocytosis
- PMID: 30578871
- PMCID: PMC7117880
- DOI: 10.1016/j.jaci.2018.10.068
IFN-γ and CD25 drive distinct pathologic features during hemophagocytic lymphohistiocytosis
Abstract
Background: Inflammatory activation of CD8+ T cells can, when left unchecked, drive severe immunopathology. Hyperstimulation of CD8+ T cells through a broad set of triggering signals can precipitate hemophagocytic lymphohistiocytosis (HLH), a life-threatening systemic inflammatory disorder.
Objective: The mechanism linking CD8+ T-cell hyperactivation to pathology is controversial, with excessive production of IFN-γ and, more recently, excessive consumption of IL-2, which are proposed as competing hypotheses. We formally tested the proximal mechanistic events of each pathway in a mouse model of HLH.
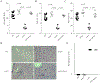
Methods: In addition to reporting a complete autosomal recessive IFN-γ receptor 1-deficient patient with multiple aspects of HLH pathology, we used the mouse model of perforin (Prf1)KO mice infected with lymphocytic choriomeningitis virus to genetically eliminate either IFN-γ production or CD25 expression and assess the immunologic, hematologic, and physiologic disease measurement.
Results: We found a striking dichotomy between the mechanistic basis of the hematologic and inflammatory components of CD8+ T cell-mediated pathology. The hematologic features of HLH were completely dependent on IFN-γ production, with complete correction after loss of IFN-γ production without any role for CD8+ T cell-mediated IL-2 consumption. By contrast, the mechanistic contribution of the immunologic features was reversed, with no role for IFN-γ production but substantial correction after reduction of IL-2 consumption by hyperactivated CD8+ T cells. These results were complemented by the characterization of an IFN-γ receptor 1-deficient patients with HLH-like disease, in whom multiple aspects of HLH pathology were observed in the absence of IFN-γ signaling.
Conclusion: These results synthesize the competing mechanistic models of HLH pathology into a dichotomous pathogenesis driven through discrete pathways. A holistic model provides a new paradigm for understanding HLH and, more broadly, the consequences of CD8+ T-cell hyperactivation, thereby paving the way for clinical intervention based on the features of HLH in individual patients.
Keywords: CD25; CD8(+) T-cell hyperactivation; IFN-γ; hemophagocytic lymphohistiocytosis.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICTS OF INTEREST DISCLOSURES
The authors declare no conflicts of interest.
Figures
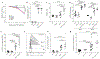




References
-
- Janka GE, Lehmberg K. Hemophagocytic syndromes - An update. Blood Rev 2014;28:135–42. - PubMed
-
- Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematol Am Soc Hematol Educ Progr 2013;2013:605–11. - PubMed
-
- Allen CE, McClain KL. Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematol Am Soc Hematol Educ Progr 2015;2015:177–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

